Covid: Bolsonaro hails suspension of Chinese vaccine trial
- Published
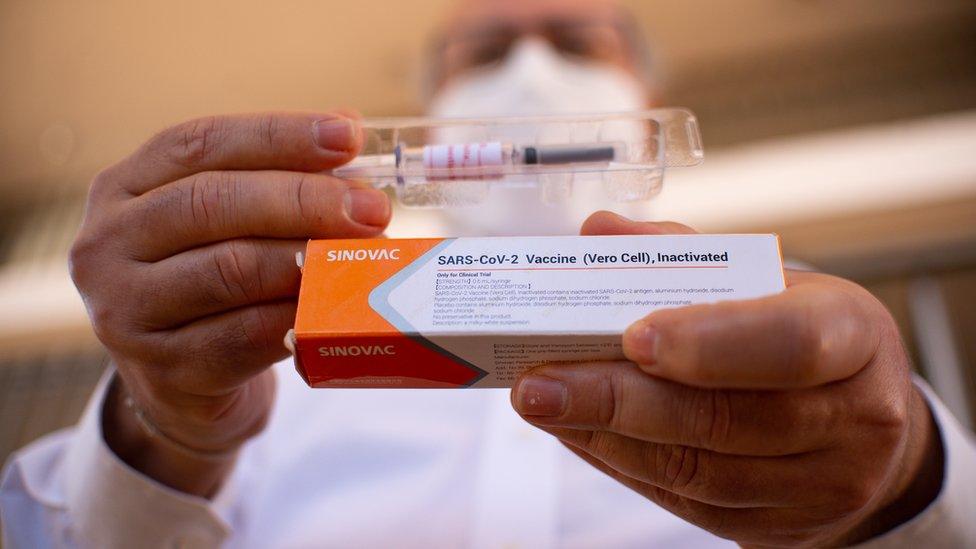
China's Sinovac vaccine has been administered in late-stage trials in Brazil, Indonesia and Turkey
President Jair Bolsonaro has declared the suspension of a Brazilian trial for a Chinese covid vaccine as a "victory".
The trial was halted after a "severe adverse incident" - reported to be the death of a volunteer.
Brazilian health regulator Anvisa said the incident had taken place on 29 October but gave no further details.
However the head of the institute conducting the trials has told local media the person's death was not linked to the medical trial itself.
'Another victory'
President Bolsonaro has long criticised the vaccine because of its Chinese links and said it would not be purchased by his country. He has also engaged in a political fight with the governor of São Paulo, Joao Doria, who has publicly backed the trial.
Writing on Facebook, the president said the halt of the CoronaVac trial was "another victory for Jair Bolsonaro".
The vaccine, developed by Chinese firm Sinovac Biotech, is one of several in final-stage testing globally. Sinovac says it is "confident in the safety of the vaccine".
The firm has already been using it to immunise thousands of people at home in an emergency use programme.
Brazil has been one of the countries worst affected by coronavirus, recording more than 5.6m confirmed cases - the third highest tally in the world after the US and India - and nearly 163,000 deaths, according to data collated by Johns Hopkins University.
Why was the trial halted?
On Monday Anvisa said it had "ruled to interrupt the clinical trial of the CoronaVac vaccine after a serious adverse incident".
It did not reveal what had happened, nor where it had taken place. Late-stage trials for the Sinovac vaccine are also being conducted in Indonesia and Turkey, but neither of these countries have announced a suspension.
Indonesia's state-owned Bio Farma said on Tuesday that its own Sinovac vaccine trials were "going smoothly", according to Reuters news agency.
Dimas Covas, head of the Butantan institute conducting the trials, told local media that the trial's suspension was related to a death, but insisted that the death was not linked to the vaccine.
This was backed up by Jean Gorinchteyn, Health Secretary for the state of São Paulo, who told a news conference that the death was an "external event" that was not related to the vaccine.
Mr Covas said that there had been no adverse reactions to the vaccine.
"We found this Anvisa decision strange, because it is unrelated to the vaccine. There are more than 10,000 volunteers at this moment," Mr Covas told TV Cultura.
He said the suspension had caused "indignation" and that the organisers of the trials had not been consulted. In a press conference on Tuesday, Mr Covas said he hoped trials would resume soon.
Sinovac said on Tuesday that it was communicating with Brazil about the reported incident.
"We learned the head of Butantan Institute believed that this serious adverse event [SAE] is not related to the vaccine," it said in a statement. "The clinical study in Brazil is strictly carried out in accordance with GCP [Good Clinical Practice] requirements and we are confident in the safety of the vaccine."
A pause in a clinical trial is not unusual. In September, the UK paused trials for another Covid-19 vaccine after a participant had a suspected adverse reaction.
The trials for the vaccine being developed by AstraZeneca and Oxford University were resumed a few days later after regulators said it was safe to continue.
Brazil's president has been open about his preference for the vaccine being developed by AstraZeneca, saying his government would not buy a Chinese-made Covid-19 vaccine.

Covid is deeply political in Brazil

Is the decision by Anvisa based on science or politics? That's the question many people are asking here because the issue of the vaccine - indeed, Covid-19 itself - is deeply political in Brazil.
From the very start, Jair Bolsonaro played down the virus, insisting the country remain open, that coronavirus was just the sniffles. But that approach was at odds with many of the country's regional leaders - even people he used to count as friends.
João Doria is the governor of Brazil's most populous (and wealthy) state. In 2018, he backed Mr Bolsonaro to become president. But the two men have since fallen out - badly.
When Covid-19 hit, Mr Doria led the way among state governors in introducing quarantine, advising people to stay at home. And that irritated Mr Bolsonaro. The governor's backing of the Sinovac vaccine has also grated.
But it's a rivalry that won't be going away any time soon. Mr Doria is expected to throw his hat in the ring for the 2022 elections too - which is why, perhaps, Mr Bolsonaro is feeling a little bit competitive.

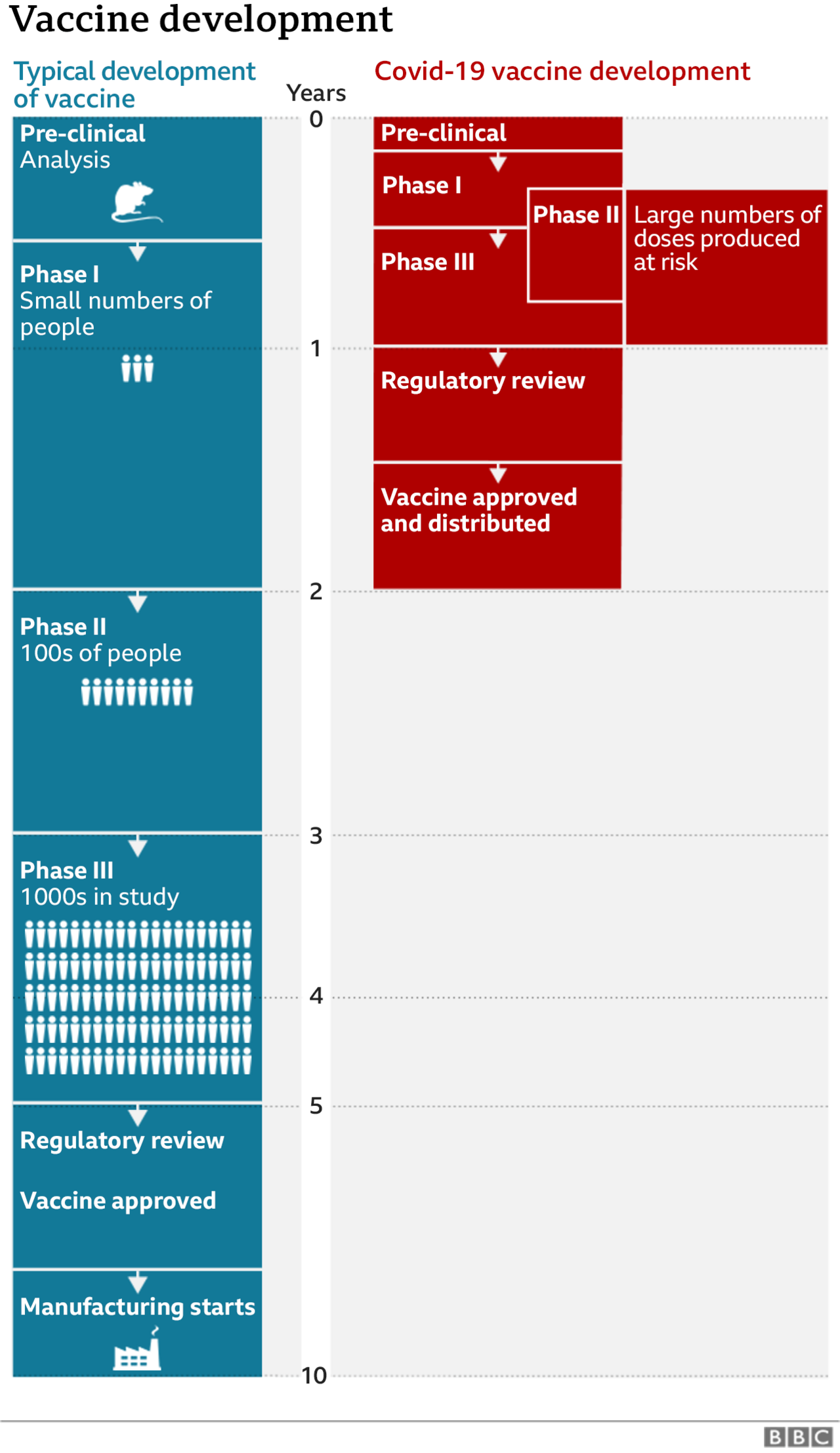
Where are we in the search for a vaccine?
CoronaVac is one of around a dozen vaccines in the final stage of testing - known as a phase 3 trial - around the world.
This is a crucial point in vaccine development, where some experimental vaccines will fail.
The news of its suspension in Brazil came shortly after a rival vaccine developer, the US pharmaceutical company Pfizer, said its own vaccine candidate had shown 90% effectiveness.
Last month the Oxford vaccine trial reviewed the death of a volunteer in Brazil, saying an assessment had revealed no safety concerns.
How has China used experimental vaccines?
Separate to the phase 3 trials being held overseas, China is also administering experimental Covid-19 vaccines at home.
CoronaVac is among three experimental coronavirus vaccines China has been using to inoculate hundreds of thousands of people under an emergency use programme.
The BBC filmed hundreds of people queuing in Yiwu, China to get the experimental Sinovac vaccine last month
Sinovac has previously said almost all of its employees and their families have received the vaccine.
And a Chinese health official earlier said that serious side effects have not been observed in clinical trials.
- Published13 August 2020
- Published17 October 2020

- Published12 September 2020

- Published21 October 2020
- Published27 August 2020
