India orders 300 million unapproved Covid jabs
- Published
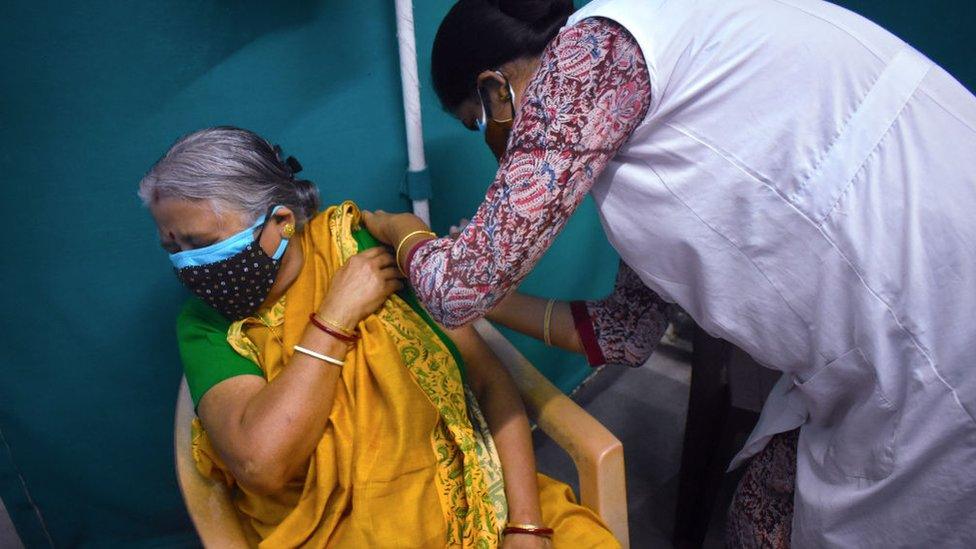
India has ordered 300 million doses of an unapproved coronavirus vaccine amid a devastating second wave.
The unnamed vaccine from Indian firm Biological E is in Phase 3 trials, and had showed "promising results" in the first two phases, the federal government said in a statement.
The $206m order is the first India has signed for a jab that has not received emergency approval.
This comes as the country struggles to speed up its lagging vaccine drive.
India has administered just over 220 million jabs so far, although much of its 1.4 billion population is now eligible for the vaccine. Less than 15% of the country has received at least one dose of the vaccination, largely because of a severe shortage of doses.
Although Covid case numbers have been dropping, India is still adding more than 100,000 news cases a day. It has recorded more than 340,000 deaths from the virus so far, but experts say the number is vastly underestimated.
India's federal government, led by Prime Minister Narendra Modi, has been criticised for not placing huge orders ahead of time with either Indian or foreign vaccine makers.

India has fully vaccinated fewer than 10% of its 1.4 billion people
India is currently giving three vaccines - Covishield, manufactured by the Serum Institute of India (SII), and Covaxin, developed by Indian firm Bharat Biotech and the government's Indian Council of Medical Research, and Sputnik V, which is developed by Moscow's Gamaleya Institute.
Compared to the single order from Biological E for 300 million doses, India brought about 350 million doses from both Covishield and Covaxin between January and May.
India's drug regulator gave Covaxin emergency approval in January before trials were completed - data on its efficacy is yet to be released.
The new vaccine from Biological E is "likely to be available in the next few months," according to the government.
Mr Modi's government is racing to shore up its vaccine stocks as Covid numbers dip, hoping to be well-prepared for what experts say is an inevitable third wave.
India's vaccine drive, which had a promising start in January, began to slow down because vaccine hesitancy crept in as cases dropped. But numbers soon rose again in a deadly second wave that saw hospitals falling short of beds and crematoriums running short of space.
Hoping to stem the tide, the government threw open the drive in May to everyone above the age of 18 but India's two vaccine makers - Serum Institute and Bharat Biotech - could not guarantee supply at that scale.
But shortages persist and have also led to vast inequalities in access with rural areas, the poor and women falling behind in the line for jabs.
Related topics
- Published14 May 2021

- Published31 December 2021
