Covid: Australia faces vaccine delays after changing AstraZeneca advice
- Published
Australia had been largely relying on AstraZeneca's vaccine
Australia's vaccine rollout is to be further delayed after local regulators advised limiting use of the AstraZeneca shot - the country's main vaccine.
On Thursday, the government said it now recommended that people aged under 50 get the Pfizer jab over AstraZeneca's.
It follows restrictions in other nations, after Europe's drug regulator found a rare blood clot risk linked to the vaccine.
The move is likely to delay a goal to vaccinate all Australians this year.
The country is already running about 85% behind schedule - it has inoculated about one million of its almost 26 million people so far.
But Prime Minister Scott Morrison said Australia could afford the delay because it had almost no community transmission of Covid-19.
On Friday, he announced that Australia had doubled its Pfizer contract to 40 million doses.
But Australia so far has only received about one million Pfizer shots - with the rest to arrive "by the end of the year", the government has said.
Australia also has a contract for 51 million Novovax vaccines, but it is yet to be approved by regulators.
Mr Morrison strongly urged people aged over 50 to continue with their vaccine, saying any risk was very rare.
"If an outbreak were to happen again... you would be putting yourself at risk if you didn't get the vaccine, because you would be exposing yourself to the more likely event of a Covid-contracted condition that could result in serious illness," he said.
Critics of Australia's rollout have condemned the government for "putting all their eggs in one basket" with AstraZeneca.
The setback upends timelines for potential border reopenings, overseas travel and economic recovery.
What is the AstraZeneca concern?
Early studies have suggested that blood clotting may occur in approximately four to six out every one million people, said Australian regulators.
They changed their advice for under-50s, after the European Medicines Agency (EMA) on Wednesday said it found rare cases of blood clots among some adult vaccine recipients. The EMA said the benefits outweighed the risks.
In the UK, the Joint Committee on Vaccination and Immunisation (JCVI) - the group of experts who advise how vaccines should be used - is now recommending healthy people under 30 be offered a different vaccine.
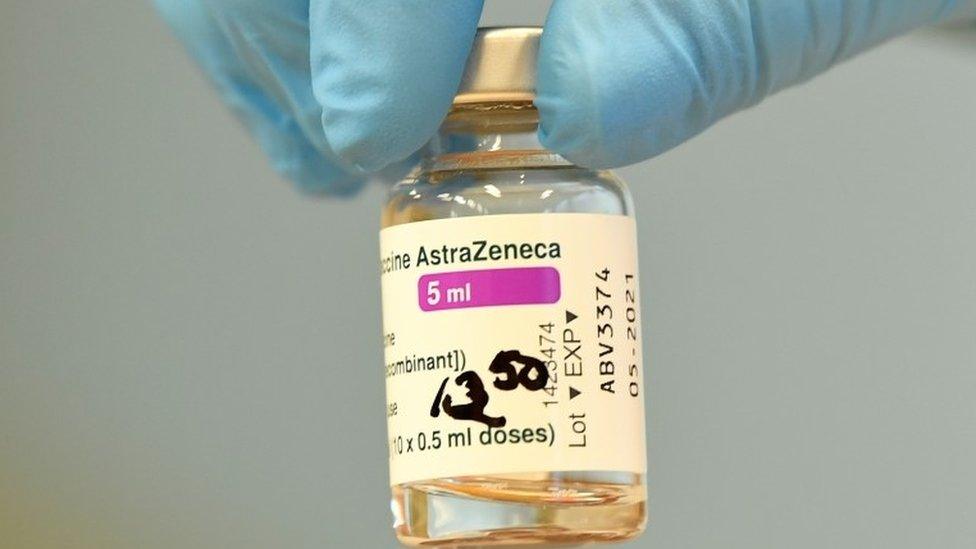
AstraZeneca said it respected the decision taken by Australian regulators. It said tens of millions of people had received its dose globally and the blood clot risk was considered a rare side effect.
"We note that the current situation Australia with very low to no community transmission of Covid-19 was a factor in this updated recommendation," it said.
Australia has reported one blood clotting case from about 420,000 AstraZeneca doses.
How will the rollout be affected?
Australia began its inoculation programme in February, later than many nations due to its low infection rates.
It is in stage two of a five-stage rollout, where the dose is being offered to more vulnerable sections of the population.
Australia now advises use of the Pfizer vaccine for people under 50
Chief Medical Officer Paul Kelly said those who had already received their first dose without side effects could "safely be given their second dose", and people over 70 would continue to get the shot.
But with about 12 million Australians aged 16-49, concerns have been raised that Australia may not have access to enough vaccinations in the short term.
The government had previously pledged to have all Australians vaccinated by October. A revised target has not been set.
Why is Australia in this situation?
Australia was relying on its AstraZeneca contract of 54 million doses, and its ability to make 50 million doses locally, to cover most of its vaccine needs.
The Labor opposition has condemned the government for failing to have more contingency plans.
Labor questioned why Australia had failed to secure supplies of the Moderna and Johnson and Johnson vaccines - which are being used in Europe and North America.
Critics have also accused the government of not being transparent about vaccine supplies.
Earlier this week, Mr Morrison blamed the rollout's delay on the European Union blocking AstraZeneca shipments from going to Australia - but this was denied by the EU.
Ros Atkins looks at how a vaccine hailed as a ‘game-changer’ has seen its reputation tarnished
Related topics
- Published7 April 2021
- Published7 May 2021
- Published7 April 2021
- Published7 April 2021

- Published31 March 2021