Covid: Pfizer-BioNTech vaccine approved for EU states
- Published
How will the new Pfizer vaccine work?
The European Union's medicines regulator has recommended the Pfizer-BioNTech coronavirus vaccine for use in the bloc's 27 states.
The European Medicines Agency (EMA) authorised the drug for the EU's nearly 448 million inhabitants after it went into circulation in the UK and the US.
Hours after the EMA's decision, the European Commission gave its own formal approval for the use of the jab.
Distribution could begin in some EU states as early as Sunday.
More than 311,000 lives, external have been lost to the pandemic across the bloc.
Fears of a new surge in cases among people gathering for the Christmas and New Year holidays have prompted partial lockdowns in countries like Germany and the Netherlands.
The threat of a new, more infectious Covid-19 variant spreading from the UK has also seen several EU states suspend travel from Britain.
How did the EMA rate the vaccine?
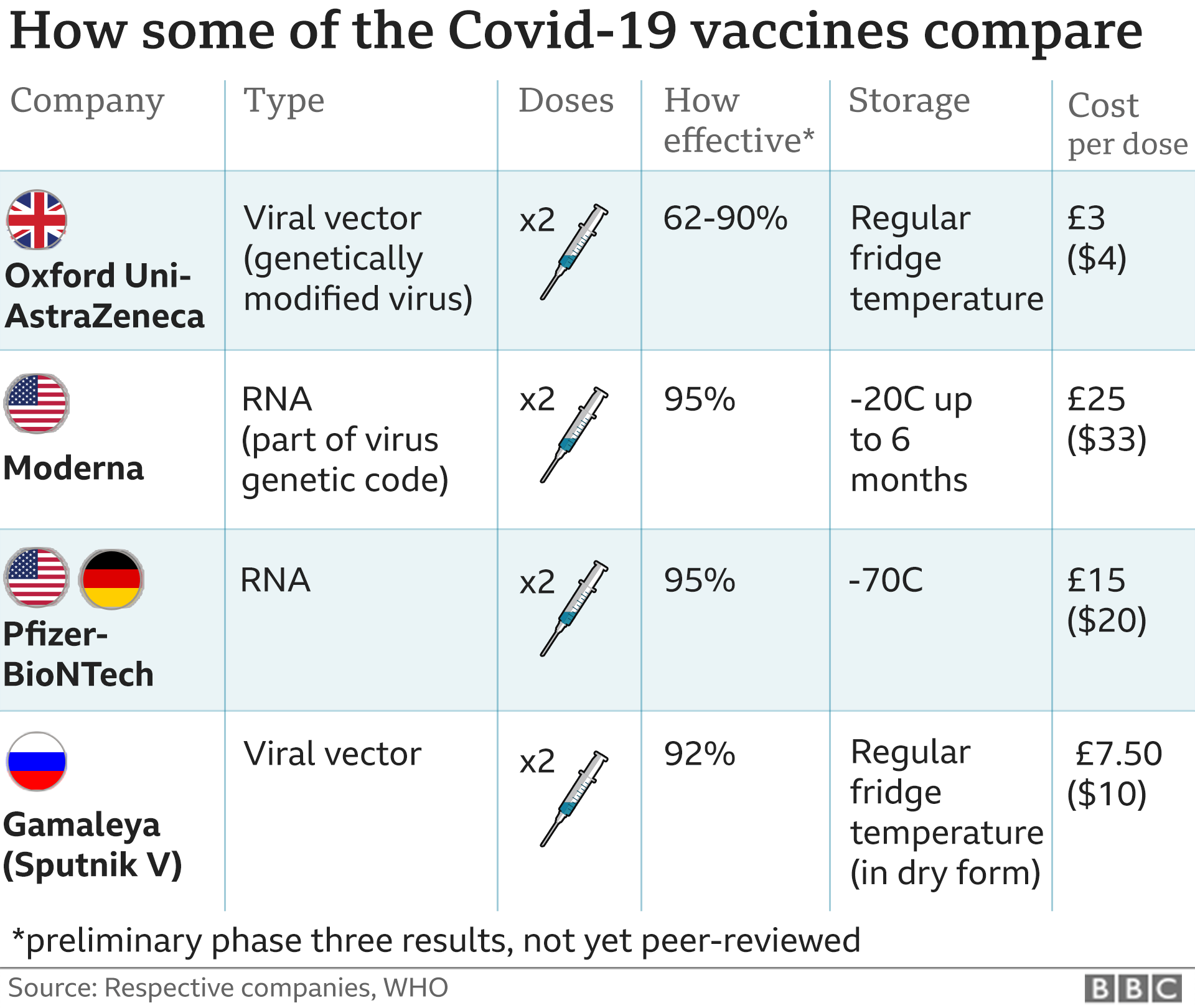
The agency said the drug had demonstrated an efficacy of 95% and could be used in people aged 16 and over.
"Today's positive news is an important step forward in our fight against this pandemic, which has caused suffering and hardship for so many," said the EMA's executive director, Emer Cooke.
Ms Cooke added: "Our thorough evaluation means that we can confidently assure EU citizens of the safety and efficacy of this vaccine and that it meets necessary quality standards.
"However, our work does not stop here. We will continue to collect and analyse data on the safety and effectiveness of this vaccine to protect people taking the vaccine in the EU."


Germany is setting up several hundred immunisation centres, and hopes to administer the first doses to its most vulnerable citizens on Sunday 27 December.
The vaccine is given as two injections, 21 days apart, with the second dose being a booster. Immunity begins to kick in after the first dose but reaches its full effect seven days after the second dose.
Most of the side effects are very mild, similar to those after any other vaccine and usually last for a day or so, said Prof Sir Munir Pirmohamed, chairman of the UK's Commission on Human Medicine expert working group, in an interview earlier this month.

Now that the Pfizer-BioNTech vaccine has been approved, EU countries can start to give the first doses of the same jab that the UK and the US is already using for mass vaccination.
The EU has ordered 300 million doses - enough for 150 million people - but only a limited number will be available immediately.
The manufacturer says it stands ready to send out the initial batches so that vaccinations could start before Christmas, but there have been some reported manufacturing issues, meaning stocks are limited, while demand is huge.
Getting the many millions of doses out to where they are needed will take months, not weeks.



CHRISTMAS: What are the Christmas restrictions around Europe?
RESTRICTIONS ACROSS EUROPE: Texting for permission to go out: Europe's lockdown rules
LATEST GLOBAL SPREAD: Tracking the global pandemic: Where has been hit hardest?
TRAVEL: What are the UK's rules?
LOOK-UP TOOL: How many cases in your area?

Related topics
- Published14 December 2020
- Published5 July 2022

- Published15 December 2020