Liberal states stockpile abortion pills after Texas court ruling
- Published

California has joined a growing number of liberal states that are stockpiling abortion pills, ahead of a looming court decision that may cut off access.
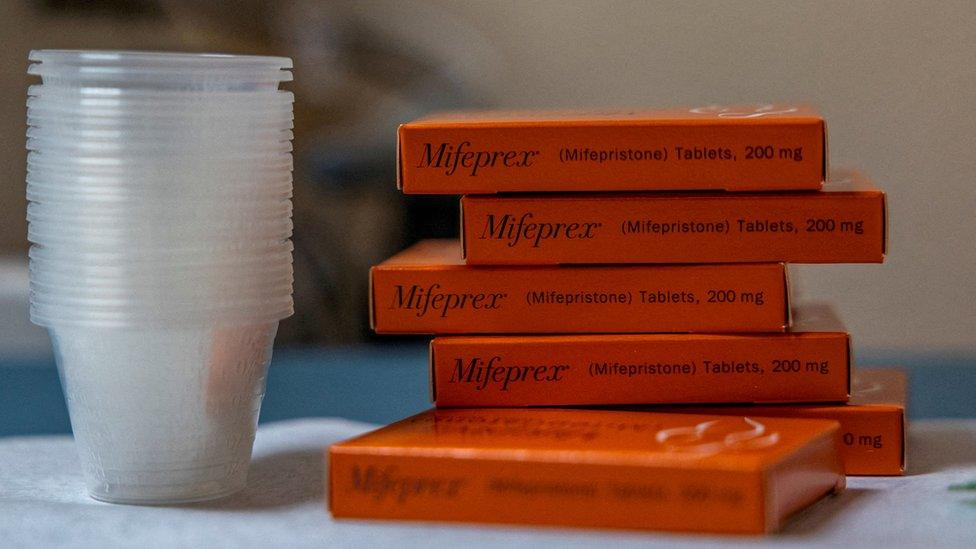
Governor Gavin Newsom said his state had moved to secure up to two million pills of misoprostol, one of the two drugs used for a medication abortion.
The action comes after a Texas judge invalidated long-standing approval for the second pill, mifepristone.
The US Department of Justice has sought a stay on the court order.
President Joe Biden on Tuesday called the Texas ruling "completely out of bounds".
So far, mifepristone remains available, but Democratic governors in Massachusetts and Washington state have also said they had secured emergency supplies of the drug amid the ongoing legal battle.
Massachusetts Governor Maura Healey said her state had ordered about 15,000 doses of mifepristone, and Washington's Jay Inslee said his state had purchased a three-year supply of the drug.
"A judge has made a politically motivated decision to override doctors, patients and medical experts and block access to critical medications," Ms Healey said on Monday. "Today, we collectively are saying loud and clear: not on our watch."
On Monday, the justice department filed an emergency motion, seeking to temporarily block the "extraordinary and unprecedented" ruling from US district Judge Matthew Kacsmaryk that invalidated decades-old approval for mifepristone.
The Biden administration lawyers have asked for a decision by 13 April - one day before the lower court's decision is set to take effect. If the justice department fails to get a stay, its lawyers will take the case to the conservative-dominated Supreme Court within hours, legal experts predict.
It is unclear how justices in either the appeals court or the Supreme Court would rule, or how exactly access to mifepristone would be restricted if the US Food and Drug Administration (FDA) was ordered to withdraw approval. Adding to the confusion was a duelling decision out of Washington, where a federal judge ordered that access to mifepristone be preserved in 17 liberal states.
The turmoil has placed a question mark over access to the drug for millions of women, and threatened the biggest blow to abortion access since the country's top court last summer ended the nationwide right to terminate a pregnancy.
More than half of abortions in the US rely on the two-pill abortion medication regimen. Mifepristone effectively stops a pregnancy, while a second drug, misoprostol, empties the uterus. Misoprostol can be taken on its own to end a pregnancy, but is considered less effective.
'Barbaric' abortion law nearly killed these Texas women
The Alliance Defending Freedom, a conservative Christian legal advocacy group that represented plaintiffs in the Texas lawsuit, argued that the FDA in its four-year approval process had ignored the potential impacts of mifepristone on the developing bodies of adolescent girls.
Its senior counsel, Erin Hawley, said the FDA had put women in harm's way "by illegally approving dangerous chemical abortion drugs, and imposing its mail-order abortion regime".
"Pregnancy is not an illness, and chemical abortion drugs don't provide a therapeutic benefit," she said.
The FDA spent four years reviewing mifepristone before it was approved, and placed mifepristone in a select category of just 60 drugs that is regulated under a system of extra restrictions, which are repeatedly re-evaluated. Its safety and effectiveness is supported by mainstream medical organisations including the American College of Obstetrics and Gynaecologists (ACOG) and the World Health Organisation (WHO).
"If the FDA can't approve this drug [mifepristone], I'm not sure what the FDA can approve," Lawrence Gostin, a professor of global health law at Georgetown University, told the BBC. "It's been on the market for over two decades, it's got an impeccable health and safety record."
Other legal experts who spoke to the BBC agreed, and said if the Texas ruling were upheld it would vastly undercut the FDA's authority, with implications for practically every drug the agency evaluates.
"It really opens the door up to virtually anybody to challenge any FDA approval," said Scott Lassman, a lawyer with over 30 years of experience in FDA law and policy.
On Monday, more than 300 pharmaceutical executives, including Pfizer CEO Albert Bourla, called for the reversal of the Texas decision, saying it was a "decision to disregard science".
With reporting from Madeline Halpert
Related topics
- Published8 April 2023

- Published13 June 2024
- Published12 July 2022