Coronavirus: Oxford-AstraZeneca vaccine trial to begin in February for children
- Published
- comments
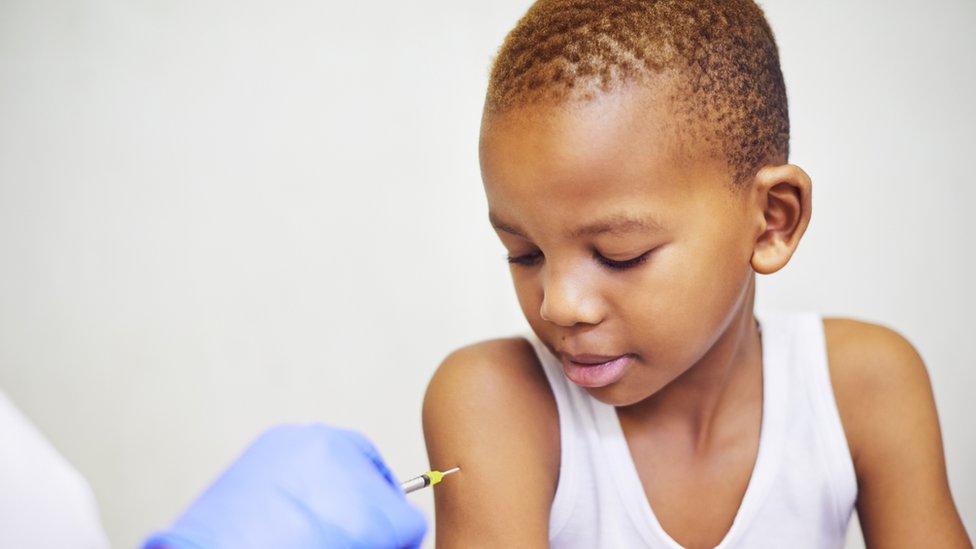
A trial to test how well the Oxford-AstraZeneca coronavirus vaccine works in children is set to begin.
Around 300 volunteers will take part in the trial, which will start at the end of February.
The scientists want to see how well the vaccine works in children aged between six and 17 years old.
Currently there are no plans to vaccinate children in the UK, but so far more than 14 million people have received one of the approved vaccines for coronavirus.

A Russian lab tests for infection with the Covid-19 coronavirus
Around 240 children taking part in the trial will get the Oxford-AstraZeneca vaccine, and the others will get a control vaccine which is not a coronavirus vaccine.
Scientists use control vaccines in trials to see what would happen if a person did not get the test vaccine, and to help them see the difference in results more clearly.
Andrew Pollard, a professor of children's medicine, and the chief investigator on the Oxford vaccine trial, said that that most children were pretty much unaffected by Covid-19 and were unlikely to become unwell with the virus.
However he also said it was important to test how the vaccine works in children, as some might benefit from having it.
The trial is taking place in four locations in UK - The University of Oxford, St George's University Hospital, London, University Hospital Southampton and Bristol Royal Hospital for Children. People living near to these locations may be asked to take part in the trial.
- Published23 November 2020

- Published7 June 2021
- Published19 November 2020