Skin cancer drug fast-tracked on NHS
- Published
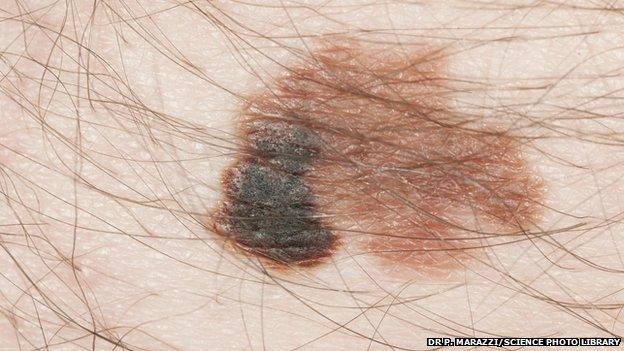
An experimental and unlicensed cancer drug has been fast-tracked to NHS patients under a new government scheme.
Pembrolizumab is a treatment for advanced skin cancer and is the first medicine to be approved through the Early Access to Medicines scheme (EAMS), launched in England last April.
The idea is to get pioneering drugs to severely ill patients much sooner.
Drugs signed off through EAMS have been scrutinised by regulators, weighing the risks and benefits.
A green light by the Medicines and Healthcare products Regulatory Agency (MHRA) means doctors anywhere in the UK can prescribe the drug in question before normal licensing procedures - which can take years - are complete.
Melanoma
Melanoma is the sixth most common cancer in the UK and kills more than 2,000 people in Britain each year.
Damage to the skin by the sun's harmful UV rays increases your risk of developing this cancer.
While advanced melanoma that has spread to other parts of the body may not be curable, targeted treatment can ease symptoms and may extend life.
Clinical trials of pembrolizumab, which is injected into the bloodstream, suggest it has great promise for treating advanced disease.

Early promise
The treatment is considered a "next generation" drug in cancer care, stimulating the body's immune system to fight the disease.
It blocks a biological pathway that the cancer uses to disguise itself from attack.
Clinical trials are still under way, although the drug has already received a licence in the US for treating advanced melanoma.
Until now, UK patients would only have been able to get pembrolizumab if they were on a clinical trial.
Another government scheme in England, known as the The Cancer Drugs Fund, external, pays for cancer drugs that have already been licensed but not yet approved by the NHS watchdog NICE.
MHRA chief executive Dr Ian Hudson said: "We are delighted to issue the first positive Early Access to Medicines Scheme scientific opinion.
"The scientific opinion describes the risks and benefits of the medicine and the context for its use, supporting the prescriber and patient to make a decision on whether to use the medicine before its licence is approved."
Emma Greenwood of Cancer Research UK said: "Pembrolizumab is another example of the great progress we've made in developing immune treatments for cancer, so it's encouraging to see it being made available to patients earlier.
"NICE and the Cancer Drugs Fund only look at licensed drugs, so it's a step in the right direction in terms of patients getting access to new treatments faster. With this approach, relevant data will be collected and patients are closely monitored.
"We look forward to seeing whether it can be replicated with other promising drugs."
- Published14 March 2014
- Published3 June 2014