Meningitis B vaccine extension rejected
- Published
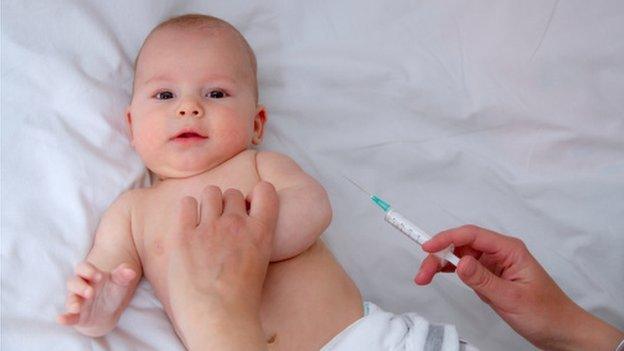
Babies are most at risk of meningitis B infection
Health campaigners say they are "hugely dismayed" that the meningitis B vaccine will not be extended to all children in the UK under the age of two.
It was introduced across the UK last year for babies aged up to 12 months.
But experts from the Joint Committee on Vaccination and Immunisation (JCVI) said there was not enough of the vaccine for a catch-up programme.
A petition calling for older children to be vaccinated was signed by more than 800,000 people earlier this year.
It was launched after pictures of Faye Burdett, who died of meningitis B in February, aged two, had been published by her family.
Under-ones are, who have been offered the vaccination since September 2015, are at the highest risk of meningitis B infection.
'Insufficient supply'
The JCVI experts concluded it would not be cost-effective to offer the vaccination to children between two and 11, because of the low levels of meningitis B in older children.
They did consider whether to support a catch-up programme for children aged up to 23 months before the coming winter.

Faye Burdett was taken to an accident and emergency unit with a rash on her forehead but died days later
But, in minutes from the committee's June meeting,, external the JCVI said there was an "insufficient supply of the vaccine to offer it to children in this age group without jeopardising stocks of the vaccine set aside for the existing NHS immunisation programme".
It said it was "acutely aware" that wider use of the vaccine could "potentially prevent further tragic losses of life" and that there was "significant public opinion" supporting such use.
The charities Meningitis Research Foundation (MRF) and Meningitis Now said they were "greatly disappointed" by the JCVI's decision, saying the risk to children aged 13 to 24 months was "only slightly less" than for younger children, and a positive recommendation "could have saved lives".
Vinny Smith, chief executive of the MRF, said: "Vaccinating children under two-years-old... would have made a significant, life-saving difference to vulnerable members of our families, so we are extremely disappointed with the JCVI's conclusion.
"This is a significant opportunity missed to save young lives from this dreadful disease this winter.
"It is regrettable that vaccine supplies to protect these children cannot be secured in time for this year's meningitis season without jeopardising meningitis B vaccinations for younger children who run an even greater risk, despite the renewed availability of vaccine for the private market."
Meningitis Now chief executive Liz Brown said: "We are hugely dismayed by today's decision, but will continue to campaign passionately for all children under the age of five to receive this lifesaving vaccine."
- Published2 March 2016

- Published18 February 2016
- Published2 March 2016