Some women need a hysterectomy after sterilisation device Essure
- Published

Laura Linkson says she felt unable to look after her children because of the pain caused by the implant
A number of women are having to undergo hysterectomies to remove a sterilisation device used on the NHS, the Victoria Derbyshire show has found.
The Essure implant is used to permanently sterilise women, but can cause side effects and complications.
One woman - who later had her uterus removed - said she was left suicidal due to the "unbearable" pain, and felt she was a burden to her family.
The manufacturer says Essure is safe and the benefits outweigh the risks.
The sale of the implants in the EU was temporarily suspended this month.
Manufacturer Bayer has asked hospitals in the UK not to use the device during this time.
'Painful to move'
Laura Linkson, who was fitted with the Essure device in 2013, said the pain left her suicidal.
"The device was sold to me as a simple and easy procedure. I was told that I'd be in and out of the doctor's office in 10 minutes and that there'd be no recovery time.
"I went from being a mum who was doing everything with her children, to a mum that was stuck in bed unable to move without pain, at some points being suicidal.
"I felt like I was a burden on everyone around me," she added.

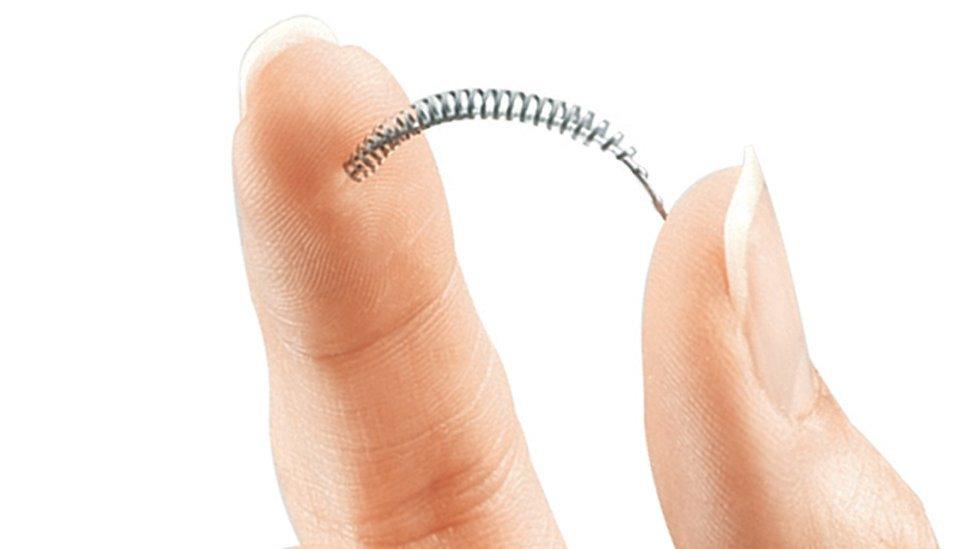
The coil is inserted into the fallopian tubes
The small coil implants, which are made of nickel and polyester (PET) fibres, are used as a sterilisation device to stop eggs reaching the womb.
They are inserted into the fallopian tubes where they trigger inflammation, causing scar tissue to build up and eventually block the tubes, known as a hysteroscopic sterilisation.
They can cause intense pain, and some women are thought to react badly to the nickel and plastic.
Because of the way the coils attach to the fallopian tubes, the only way to take them out is to remove a woman's fallopian tubes and often her uterus.
In other cases the device has been found to perforate a fallopian tube and fallen out, embedding itself elsewhere in the body.
Victoria Dethier was implanted with Essure in 2012 and for three years could not work out why she felt so unwell.
Victoria Dethier had a hysterectomy to remove the device
"There were moments where I couldn't get out of bed I was in so much pain. It felt like I was dying, like something was killing me from the inside," she said.
She thinks her body was reacting to the PET fibres designed to cause inflammation.
She had a hysterectomy to remove the device in 2015.
"Straight away there was a difference, I'd experienced a horrible taste in my mouth and that had gone," she explained.
"I'd lost a lot of hair and that came back within 12 months, it was incredible."
'We need acknowledgement'
The medicines and healthcare products regulatory agency (MHRA), external has been criticised for not responding to the increasing evidence regarding the device.
In 2015, a study published in the British Medical Journal (BMJ) suggested that women who had a hysteroscopic sterilisation were 10 times more likely to need follow-up surgery than those who had a traditional sterilisation - 2.4% of those surveyed, as opposed to 0.2% amongst those having a standard sterilisation.
In the US more than 15,000 women have reported problems to the US Food and Drug Administration (FDA), including pain, allergic reactions and "migration of device".
Carl Heneghan, from the Centre for Evidence-Based Medicine at Oxford University, has criticised the regulator's failure to act on such findings.
"How much evidence do you need to say let's withdraw this from the market?" he asked.
Victoria Dethier is angry that she and so many other women feel they have been ignored.
"No-one is listening to us," she said.
"There are many women coming forward... we need to be acknowledged."
'No long-term evidence'
The full extent of the problem in the UK is not known.
The MHRA rejected the Victoria Derbyshire programme's Freedom of Information request asking how many women have reported problems.
The NHS does not have figures for the total number of women who have been fitted with Essure, or who have had it removed.
However, the clinical trial that led to the device being approved has been criticised for not considering the long-term effects of the implants.
"The trial... only followed up women for one year, so nobody has a real understanding of what happens with this device after two years, three years, five years," Mr Heneghan explained.

Consultant obstetrician Ben Peyton-Jones says Essure implants can be safe when used correctly
Some women who have experienced problems say they were not informed about the risks.
But Ben Peyton-Jones, a consultant obstetrician and gynaecologist, said the device should still be used in some instances.
"I think it has a place for women who can't have keyhole surgery and who are explained the risks very carefully," he said.
"When used correctly, according to the manufacturer's guidance and in trained hands, it is safe."
The sale of Essure implants in the EU has now been suspended for further investigation.
Hospitals have been asked by Bayer not to use their existing stocks during this time.
It is a voluntary request and up to individual trusts to decide what to do.
The company said that independent reviews of Essure had concluded that the benefits outweighed the risks.
"Patient safety and appropriate use of Essure are the greatest priorities for Bayer, and the company fully stands behind Essure as an appropriate choice for women who desire permanent contraception," it added in a statement.
"Many women with Essure rely on this form of contraception without any side effects."
The MHRA said it had no evidence to suggest this product was unsafe, and that the recent suspension did not suggest any increased risk to patient safety.
It said it was important for healthcare professionals to discuss the risks with patients before a procedure.
Watch the BBC's Victoria Derbyshire programme on weekdays between 09:00 and 11:00 on BBC Two and the BBC News channel.
- Published14 October 2015
- Published4 October 2015
