Immediate stop to NHS mesh operations
- Published

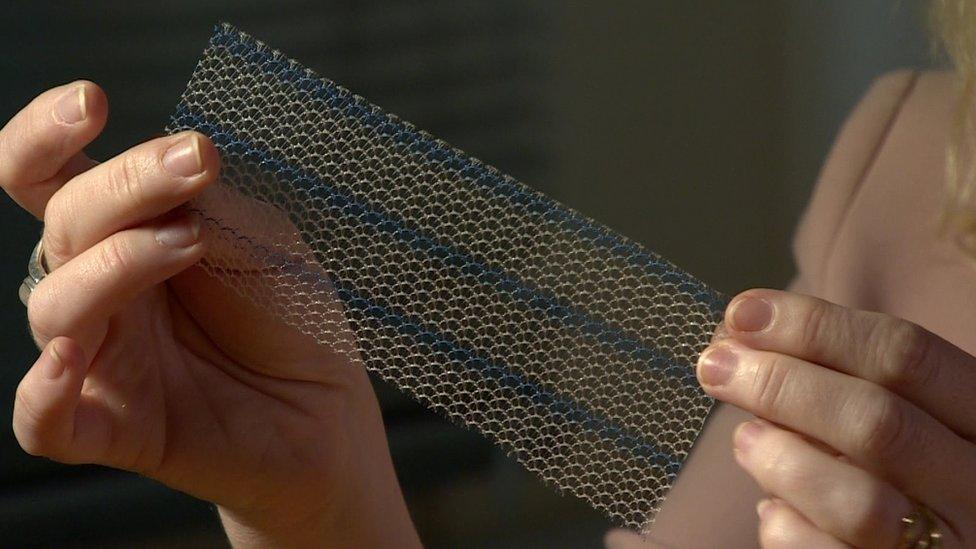
The mesh implants are used to ease incontinence and to support organs
NHS England is putting an immediate curb on mesh operations after safety concerns.
It has accepted the advice of a new review looking at harm reported by women who received the treatment for stress urinary incontinence.
The review's chair, Baroness Julia Cumberlege, said she was "appalled at the seriousness and scale of the tragic stories" that her team had heard.
Many women say the implants caused them agony by cutting into tissue.
Some say they have been left with life-changing injuries.
Claire Cooper, who had the implants, mistakenly had a hysterectomy after doctors were unable to diagnose what was causing her pain. She was left feeling suicidal and unable to have sex.
Claire Cooper was left feeling suicidal
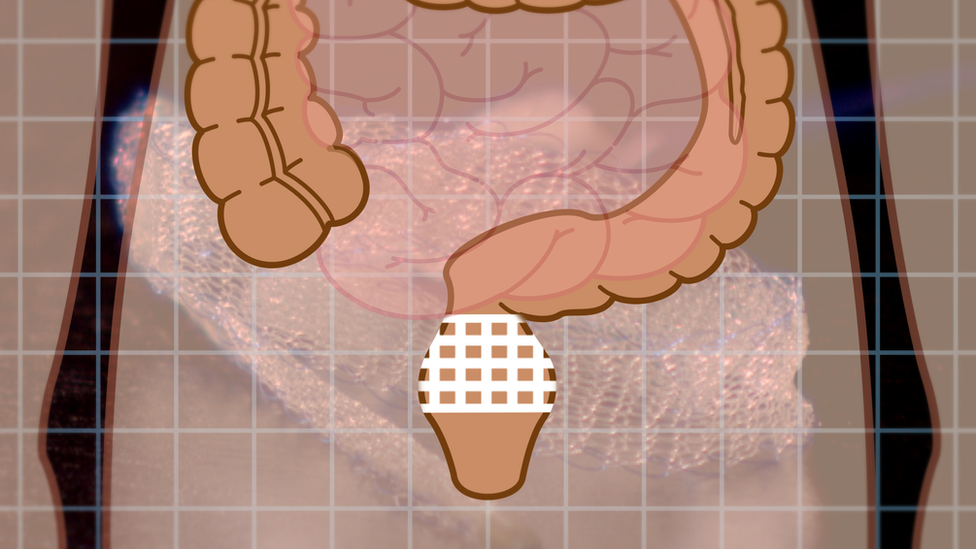
It is estimated that more than 100,000 UK women have had a mesh fitted. The net-like fabric can be attached into the wall of the vagina to act as a scaffold to support organs, such as the bladder, to keep them in the right place to help manage incontinence or another condition called prolapse.
Most patients suffer no ill effects, NHS England says.
Not a total ban
England's Chief Medical Officer, Prof Dame Sally Davies, said mesh would remain a treatment of last resort for some: "Carefully selected patients will continue to have access in discussion with their consultant."
Baroness Cumberlege said the independent review found no evidence on the benefits for treating urinary incontinence that would outweigh "the severity of human suffering caused by mesh complications".
"My team and I are in no doubt that this pause is necessary. We must stop exposing women to the risk of life-changing and life-threatening injuries. We must have measures in place to mitigate the risk, and those are sadly lacking at the moment.
"At this stage in our review we are not recommending a ban, but a halt to procedures."
The pause can be lifted if certain checks and measures are met by March 2019, says the review team.
This includes keeping a register of every procedure and any complications.
To date, it's still unclear how many women have been adversely affected by mesh. The government is carrying out an audit to try to find out.
The health watchdog NICE has already recommended that vaginal mesh operations for treating organ prolapse should largely be stopped in England.
The use of vaginal mesh to treat urinary incontinence was not mentioned in the draft NICE guidelines, however.
A number of Scottish health boards have already stopped using mesh implants altogether, and in Wales the procedures are seen as a 'last resort'.
The safety review chaired by Baroness Cumberlege is also looking at concerns about a hormone pregnancy test called Primodos and an epilepsy drug called sodium valproate, which have both been linked to birth defects.
Mesh used for bowel patients (rectopexy) has not been included in the temporary suspension.
- Published17 April 2018
- Published6 July 2018
- Published18 April 2017
