How antibiotic resistance could take us back to the 'dark ages'
- Published
Scientists are working on a new antibiotic that has produced promising results in early trials. Medicine will need more of these in coming years if increasing bacterial resistance is not to pose a very serious threat to human health.
When they were introduced, in the 1940s, antibiotics were hailed as a "wonder drug". But there are major concerns that over-prescription has led to increasing resistance to the drugs.
Last week, the Chief Medical Officer for England, Prof Dame Sally Davies, went as far as to say we were "at risk of putting medicine back in the dark ages" - but why has this happened?
What are antibiotics?
Scottish chemist Alexander Fleming discovered the first true antibiotic, penicillin, in 1928, as a mould on a petri dish that inhibited the growth of bacteria. His discovery revolutionised the treatment of certain types of bacterial infection, saving countless lives in the process.
Antibiotics fight bacteria in a number of ways, including by killing them or preventing them from spreading.
However, they also have a major weakness.
Resistance
Antibiotics are very effective at killing most, but not all, bacteria. Some bacteria acquire genes that protect them from the drug's attack.
They survive treatment and reproduce themselves, spreading the key genes more widely so the drug becomes ever less effective.
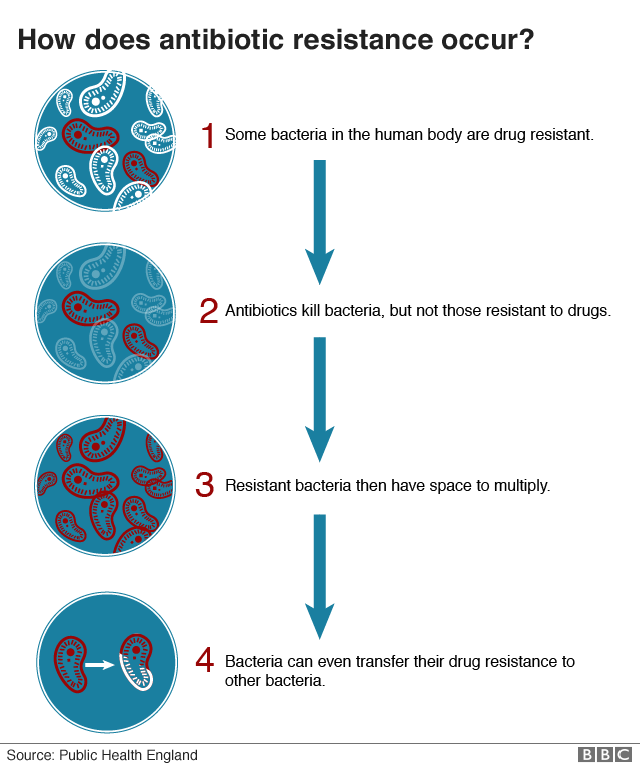
If somebody is infected by these drug-resistant bacteria, then it can become harder to deploy antibiotics to treat them successfully.
At present other types of antibiotic might do the trick but the options are starting to narrow as bacteria develop the ability to block more than one drug.
In the past four years in England, there has been a 35% increase in antibiotic-resistant blood infections. This is because there have been greater efforts by clinicians and other health professionals to identify sepsis cases.
However, even though the absolute number of infections being detected has increased, the relative proportion of blood infections resistant to antibiotics has remained stable.
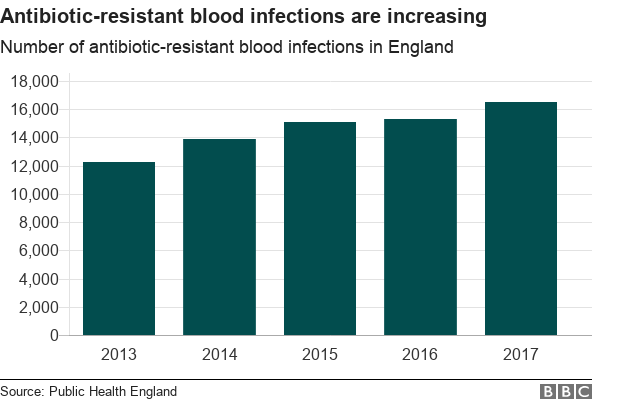
From the point of view of health experts, a key to reduce antibiotic-resistant blood infections is to ensure that resistant bacteria cannot become prevalent in the first place.
A recent report, external highlights that without effective antibiotics, life-threatening infections linked to operations such as hip replacements and Caesarean sections could increase.
Overprescribing
Since 2013, Public Health England has actively been campaigning to reduce the amount of antibiotics taken by patients.
They say that over-prescription of antibiotics is a major cause of the increase in resistance to them. This is because the more antibiotics are used are used, the less effective they become.
In addition, GPs have been prescribing antibiotics to patients who are not infected with bacterial diseases, even though they will never work.
Antibiotic consumption in England has decreased by about 5% since 2013 - but the average daily dose per 1,000 people varies across the country.
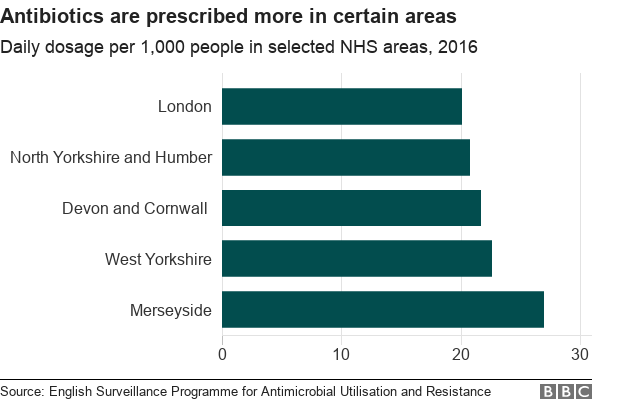
Public Health England points out that antibiotic resistance and prescription are "inextricably linked - areas with high levels of antibiotic prescribing also have high levels of resistance".
Its campaign involves ensuring all areas have access to best practices for using antibiotics - in particular in community care, as 90% of antibiotics are prescribed by GPs.
They want to see patients and carers educated about the inappropriate use of antibiotics and greater efforts by health professionals in preventing infections in the first place.
And in 2016 the government called for a 50% reduction in the inappropriate prescription of antibiotics by 2021.
About half of all patients with a cough or cold leave their GP's surgery with a prescription for antibiotics.
And there are concerns that patient expectations are driving the problem.
Recent research suggests 38% of people expect to be prescribed antibiotics when they go to the doctor.
So efforts are now being made not only to reduce the number of antibiotics prescribed but also for GPs not to prescribe antibiotics for conditions that will naturally clear up by themselves after a few days.
How does the UK compare?
Over-prescription is not a problem unique to the UK.
The European Surveillance of Antimicrobial Consumption Network has called the spread of drug-resistant bacteria a "public health threat", with estimates suggesting that 25,000 people die in Europe from linked infections every year.
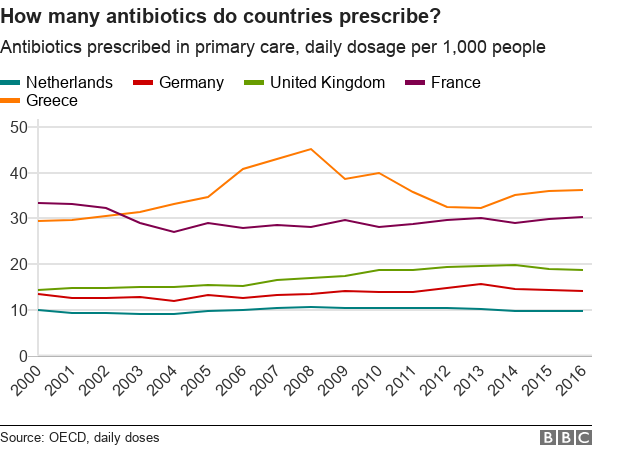
Currently, the UK has below average consumption compared with other EU countries.
Efforts to mitigate future costs related to the problem have led countries with high consumption to learn from others - in particular in northern Europe - who consume fewer antibiotics.
The global picture

One of the biggest global concerns regarding antibiotics is over drug-resistant tuberculosis (TB).
According to the World Health Organisation (WHO), TB remains the world's deadliest infectious disease.
Treatment has been effective, with an estimated 54 million lives saved through diagnosis and treatment between 2000 and 2017.
Even so, and with the global incidence of TB falling at about 2% per year, it remains one of top 10 causes of death worldwide.
In 2017, 10 million people fell ill with TB and 1.6 million died from the disease, mainly in developing countries.
The WHO says that 490,000 people have multidrug-resistant tuberculosis (MDR-TB), when bacteria do not respond to the two most powerful first-line anti-TB drugs, isoniazid and rifampicin.
Second-line drugs can treat and cure MDR-TB. However, such treatment options can mean that patients have to undergo up to two years of chemotherapy involving costly and toxic medicines.
Looking to the future
It has been 30 years since a new class of antibiotic became available.
And resistance has developed to all those that do exist.
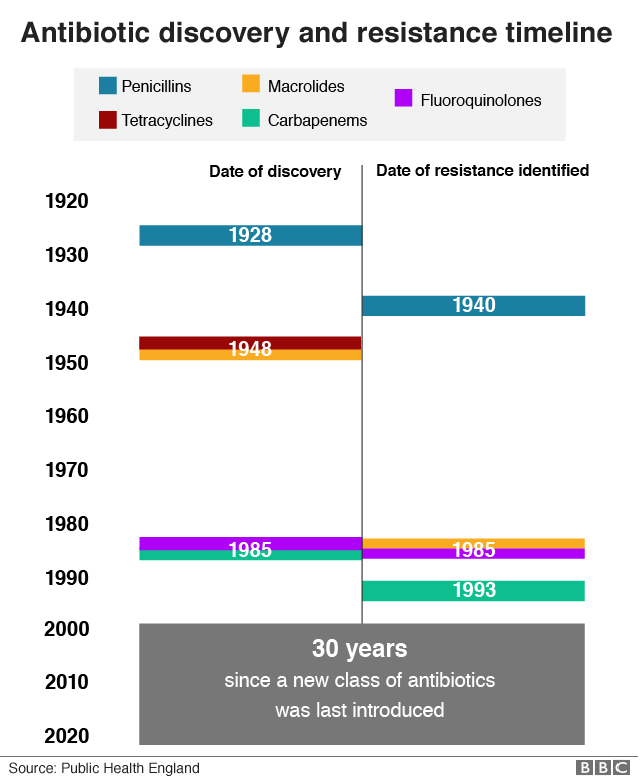
But antibiotics are expensive to produce and can take a long time to become available to clinicians and patients.
In 2017, Public Health England warned that a failure to address resistance to antibiotics, external could lead to an estimated 10 million deaths every year globally by 2050 at a cost of £66trillion in lost productivity to the global economy.
That is why they, and other health organisations, are calling for a reduction in the overuse and misuse of antibiotics.