Some HRT tablets 'linked to higher blood clot risk'
- Published
Women taking certain types of hormone replacement therapy (HRT) tablets could be more at risk from serious blood clots - although the overall risk is low, BMJ research suggests.
It found tablets containing equine oestrogen were linked with a slightly higher risk than other tablets.
And patches and gels for HRT were the safest but were underused.
GPs' leaders said HRT treatments were tailored to meet the needs of individual patients.
They said women should not panic or stop taking HRT. Instead, they should discuss any concerns at their next routine GP appointment.
HRT is used to relieve symptoms of the menopause such as hot flushes and night sweats, by replacing hormones that are at a lower level.
The treatments come in a number of different forms, including tablets, gels, cream and patches.
Most experts agree that HRT is a good and safe treatment - but there are some small potential risks, as NHS UK advice, external explains.
These include a small increased risk of certain serious health problems, such as blood clots and breast cancer.
Underused treatments
This study, external, by University of Nottingham researchers, said the increased risk of taking HRT tablets was equivalent to nine extra cases of blood clots per 10,000 women per year.
The study looked at the prescription records of 80,000 women aged 40-79 who had developed blood clots and compared them with records of 390,000 women who had not.
For tablet treatments, the risk was found to differ for two types of oestrogens.
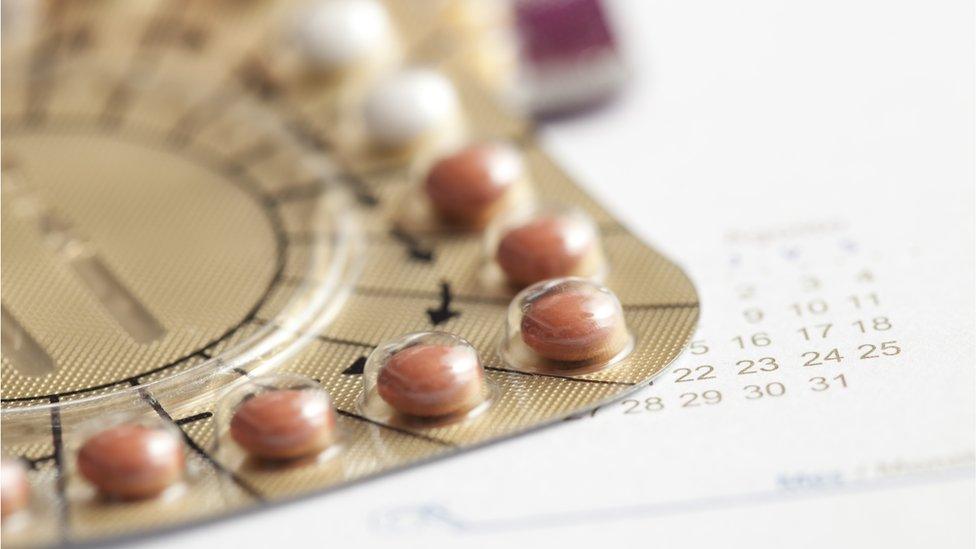
The risk of blood clots was 15% higher for the treatments containing oestrogen manufactured from horse urine than for the synthetic oestradiol, for both single and combined hormone treatments.
But there was no such risk for women using gels, patches or creams for HRT - also called transdermal treatment.
The study said this was the safest type of HRT and yet it appeared to be underused, with just 20% of prescriptions for this type of therapy.
According to the Royal College of GPs, most local NHS groups suggest prescribing tablets as a first-line treatment, unless medical issues suggest otherwise.
'Don't panic'
Dr Yana Vinogradova, from Nottingham's school of medicine, said: "Our study has shown that, for oral treatments, different tablets are associated with different risks of developing blood clots, depending on the active components.
"It has also confirmed that risks of thrombosis for patients using HRT treatments other than tablets [patches or gels] is very low."
She added: "Our findings are particularly important information for women who require HRT treatment and are already at increased risk of developing blood clots."
Prof Helen Stokes-Lampard, who chairs the Royal College of GPs, said the observational study was "interesting" but could not prove that cases of blood clots - or deep vein thrombosis - had been caused by the tablets.
"As such, it is essential that more research is conducted in this area and taken into account as new clinical guidelines are updated and developed," she said.
She said current practice was to prescribe the lowest possible dose of HRT for the shortest possible time.
This happened after "a comprehensive discussion between the GP and their patient", she said, when treatments were tailored to meet the best interests of each individual.
"It's important that patients don't panic or stop taking HRT as a result of reading about this study but instead discuss their concerns at their next routine GP appointment or seek advice from a reputable website like NHS Choices, external," Prof Stokes-Lampard said.
Safety information
Dr June Raine, from the Medicines and Healthcare products Regulatory Agency (MHRA), said details of the risks were included in the prescribing information for all HRT products.
"Previous studies have suggested a lower risk of blood clots with transdermal patches than with oral tablets, but these studies have included too few women using transdermal patches to allow firm conclusions to be drawn."
She added: "Any new significant information on the efficacy or safety of HRT tablets will be carefully reviewed and the information provided to healthcare professionals, and patients will be updated if appropriate."
- Published10 March 2015

- Published13 February 2015

- Published28 April 2014
