'Lack of evidence' holding back cannabis medicines
- Published
- comments
Anthony and Tannine travel to the Netherlands to obtain cannabis oil for their child
NHS patients are not getting cannabis-based medicines because of a lack of evidence they work, according to an official review.
Multiple sclerosis patients and parents of children with severe epilepsy have been fighting for access to the drugs.
The NHS England report found hospitals also had issues affording the medicines and getting hold of a safe supply.
The report said the health service must support studies to gather evidence "as soon as possible".
The law was changed in November 2018 to allow specialist doctors to prescribe medicinal cannabis for the first time.
The decision fuelled demand from parents of children with severe epilepsy, who were having hundreds of seizures a week, as well as patients with muscle stiffness caused by multiple sclerosis.
But the demand rapidly turned to frustration as the drugs were hard if not impossible to get hold of.
Some families admit to smuggling cannabis-based medicines into the UK and spending £1,500 a month on the drugs.
The Health Secretary, Matt Hancock, ordered NHS England to investigate why the drugs were not being prescribed.
The NHS review, external said the "major hurdle" was doctors being unsure of the benefits and long-term safety of the drugs - because of a lack of randomised clinical trials.
These are the "gold standard" of medical research. And the review said the NHS must support such trials as well as look at other forms of evidence.
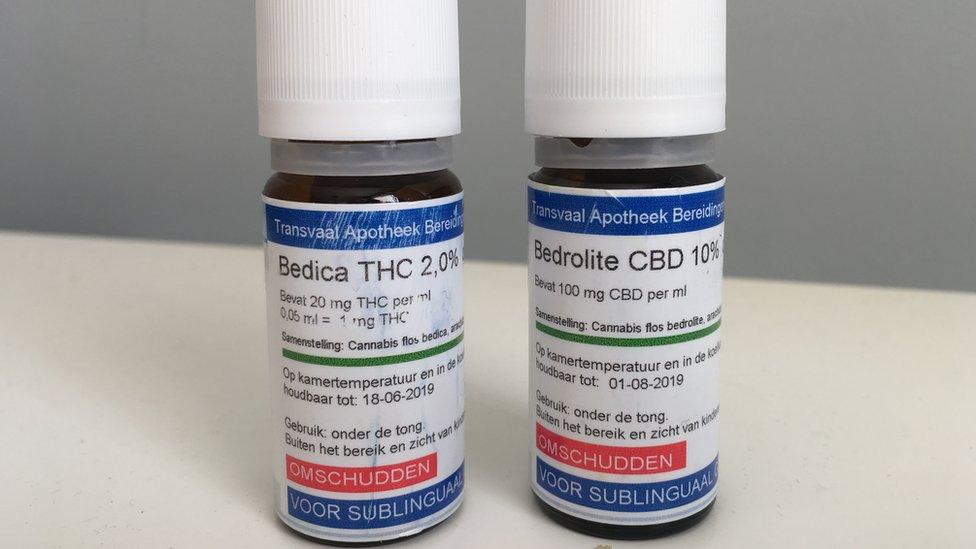
These cannabis-based medicines are not licensed in the UK but are available if prescribed by a specialist doctor
Peter Carroll, the director of the End Our Pain Campaign, said: "There are many positives in this review, in particular with regards to the evidence that can and should be taken into account relating to medical cannabis.
"But the fact remains that the families we represent are in urgent need of access to medical cannabis right now."
But even if doctors thought a cannabis prescription was appropriate, the report showed NHS funding was a problem and hospitals felt the costs were "unsustainable in the long term".
There were also concerns the drugs - which are often unlicensed - are not of high enough quality.
'Too expensive'
Meanwhile, the body that advises on best treatments, the National Institute for Health and Care Excellence (NICE), has issued guidance to doctors.
In the case of Sativex, a cannabis-based medicine for spasticity in multiple sclerosis, NICE said it was too expensive.
"We recognise that some people will be disappointed that we have not been able to recommend the wider use of cannabis-based medicinal products," said Paul Chrisp, the director of the centre for guidelines at NICE.
The MS Society and Epilepsy Action charities both said they were disappointed by the guidelines.
Follow James on Twitter, external.