Coronavirus: Why vaccines rely on volunteers
- Published

The world eagerly awaits a coronavirus vaccine, and labs are racing to develop one. Some have now reached the stage of human trials and are looking for volunteers. So what's it like to be part of a vaccine trial?
I remember very clearly my first medical trial. It was in Oxford where I was going to receive an experimental vaccine against bird flu.
This was in 2006 and at the time H5N1 avian flu was a big deal. It was a deadly virus, killing half of those it infected. That would make it perhaps 50 times more lethal than Covid-19.
So there was a need for a vaccine, and the Oxford Vaccine Group was to conduct a trial of healthy volunteers.
I didn't hesitate about sticking my hand up, or rather, rolling up my sleeve. After all, I rely on patients to agree to me filming them in order to illustrate some aspect of healthcare, so it was a good thing for me to experience what that's like. Very often they are taking part in a medical trial, be it for cancer, diabetes or any number of other conditions.

Naturally I was filmed while receiving the vaccine. I can remember being determined not to grimace because I didn't want to set a bad example. The immunisation takes just a few seconds, and I also had to give some blood samples the same day.
As the needle for the blood draw was about to go in, a kindly doctor said "sharp scratch" which was my cue to look into the lens and deliver a faultless "piece to camera" - the bit in most TV reports when correspondents pontificate. But with a needle in your arm you have to get it right the first time.
As my blood entered the test tube I spoke about antibodies and immunity.
I think it went fine, but I remember a recent occasion that was a nightmare. Again, I started speaking as the needle went in, and my words, thankfully, flowed out on cue. The trouble was, the red stuff did not. It was like getting blood from a stone. It took four needles, and by the end of the filming the colour had drained from my face and I was in a cold sweat.


Fergus on Covid
As the BBC's medical correspondent, since 2004 I have reported on global disease threats such as bird flu, swine flu, Sars and Mers - both coronaviruses - and Ebola. You could say I've been waiting much of my career for a global pandemic. And yet when Covid-19 came along, the world was not as ready as it could have been. Now it's here I wish, like everyone else, it would go away. Sadly, we may have to live with coronavirus indefinitely. In this column I will be reflecting on that new reality.

The bird flu vaccine trial went well. The following year it was approved for use, but bird flu never made the full jump from animals to humans, so it's not been needed.
Not so with coronavirus. To say the results of vaccine studies are eagerly awaited would be the understatement of the year. There are lives, livelihoods and whole economies depending on a successful vaccine against Sars-CoV-2, to give the virus its proper name.
More than 100 vaccines are in development around the world. Many of these may never get to human trials, but several have already reached that stage, in record time. Normally it would take years, decades even.
The Chinese government shared the genetic sequence of coronavirus on 11 January, and within weeks a team at the University of Oxford had developed an experimental vaccine. Their human trial, the first in Europe, began last month.
More than 1,000 volunteers are now part of the Oxford vaccine study. Half will get ChAdOx1 nCoV-19, as the vaccine is called, and the rest a control vaccine which protects against meningitis. The trial is "blinded" so that the researchers and the volunteers won't know which jab they are getting.
The use of a real vaccine as a control, which may result in the odd sore arm, means volunteers really won't know whether they have got the real thing. That's important, as all of them will need to keep a daily health diary for up to four weeks, and come back for several blood tests.
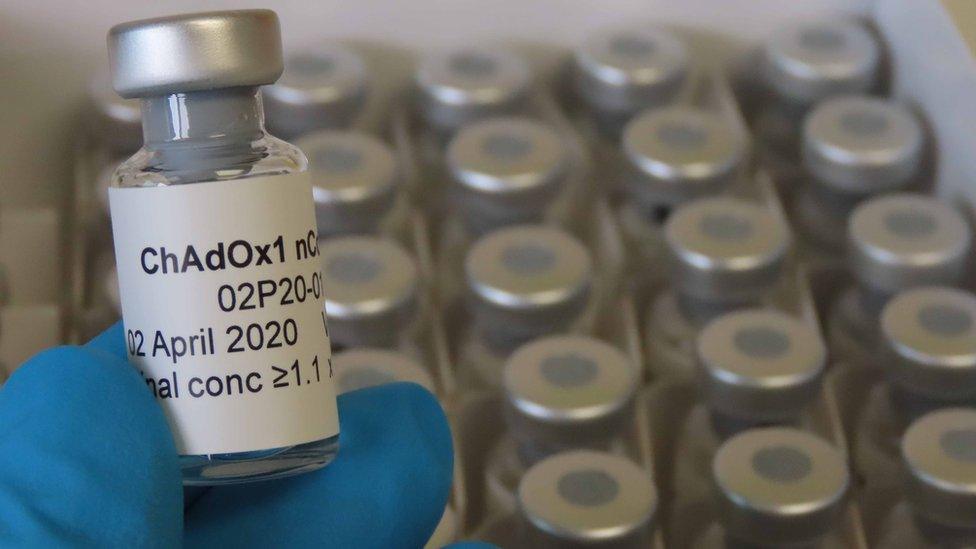
Samples of the vaccine have been made for the trial
I wanted to volunteer for the first phase of the vaccine trial, but was ineligible. It's the first time I've been too old for a clinical study - the cut-off was 55 years and I'll be 59 in September. Though the trial has now been extended to include older adults and children aged five to 12, I may still be ruled out. Anyone who already has antibodies to coronavirus is excluded, and as I explained last week, I'm pretty sure I have these. I have signed up online just in case they will have me.
Elisa Granato was the first volunteer to be injected
In order to check whether the vaccine works, it is vital that those given the jab come across the virus in their daily lives. So the team would like volunteers who have public-facing roles, especially health workers, who are more likely to be exposed to coronavirus.
You don't need all of your volunteers to get up close and personal with the virus, but it's important that some do, and in the absence of a guaranteed treatment it would be unethical to deliberately infect them.
The volunteers are all told to maintain the same social distancing as the rest of us. And remember they don't know which vaccine they have received.

Fergus holding a vial of the vaccine developed by the Oxford team
There's one big problem surrounding all of this, which is that you need a lot of virus to be circulating to know whether the vaccine protects the people who've been immunised, and at present cases are decreasing. It's reckoned about one in 1,000 people in England are currently infected, not counting cases in hospitals or care homes.
A further 10,000 volunteers are being recruited, external at sites across England, Wales and Scotland. At present there are more cases in parts of the north of England and Scotland than in Oxford.
Coronavirus vaccine: How close are you to getting one?
Volunteers are absolutely crucial to medical advances; we'd never get anywhere without them. The same goes for blood donors. With all the volunteers I've spoken to over the years there's a really strong element of giving something back. People say, "Well, it may not help me, but it'll help those coming after me."
Some years ago, I covered a typhoid study in Oxford, in which they were immunising people with a new vaccine, and then infecting them deliberately with the disease - they could do this because it can be treated with antibiotics. The volunteers had to swallow a drink that had typhoid bacteria in it, and I remember one of them saying, "Down the hatch!" before they drank it.
That vaccine is now being used in Pakistan and Zimbabwe, and has reduced cases of the disease by 80%. When I let those volunteers know recently, they were delighted. That trial was of absolutely no benefit to their daily lives, they did it purely because they felt it was the right thing to do. But with the coronavirus trial there may be some benefits for volunteers.

LOCKDOWN UPDATE: What's changing, where?
EXERCISE: What are the guidelines on getting out?
THE R NUMBER: What it means and why it matters
AIR TRAVELLERS: The new quarantine rules
LOOK-UP TOOL: How many cases in your area?

There has been much speculation about when we will get results from the Oxford vaccine trial. I've heard September, even June. The hard truth is, it's not certain when we will know. It depends on whether we get a second wave of infections.
If, and it's still a big if, the vaccine does work, hundreds of millions of doses could be made within months because of a manufacturing deal struck with the pharma giant, AstraZeneca. It says it could produce a billion doses by the end of 2021.
And the Oxford vaccine is not the only show in town. Imperial College London is developing a coronavirus vaccine which will begin human trials next month. All the researchers I've spoken to have said this is not a race to be first, but a race against the virus. It's a race we all hope they will win.
Find out more about the Oxford Vaccine trial, external
Follow @BBCFergusWalsh, external on Twitter

Read last week's column:
Antibody tests which show that you have had a Covid-19 infection are being rolled out, prioritising NHS and care staff. So what happens when you test positive? Carry on as before - and I should know.
'I was gobsmacked to test positive for coronavirus antibodies'
