Covid vaccine: Major new trial starts in UK
- Published
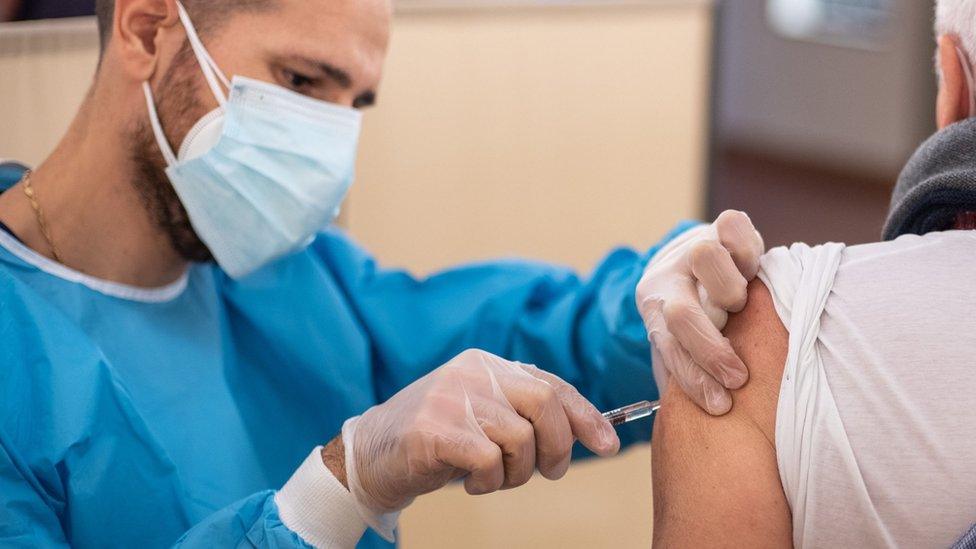
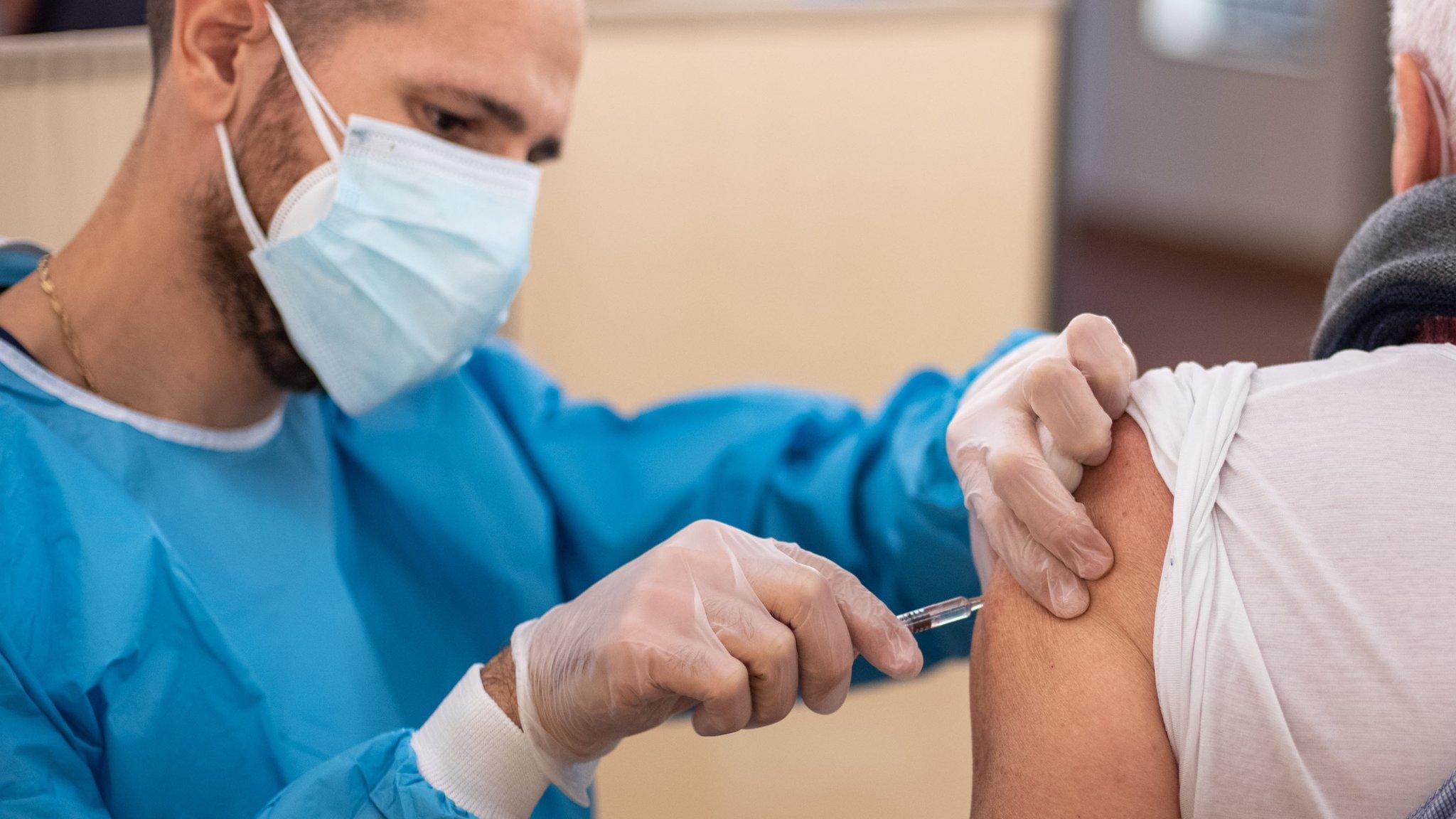
A major trial of a vaccine to protect against Covid-19 has launched in the UK - the third such trial in the country.
The jab - designed by the Belgian company Janssen - uses a genetically modified common cold virus to train the immune system.
It comes a week after preliminary results showed another vaccine offered 90% protection.
However, many types of vaccine are likely to be needed to end the pandemic.
The success of the vaccine developed by Pfizer and BioNTech has caused global excitement. However, it has not yet been approved for use and we still do not know how well it works in the elderly or how long immunity lasts.
The hunt for Covid vaccines continues as a different approach may yet be better, or better in some age groups, and one company will struggle to immunise the planet.
"It is really important we pursue many different vaccines from many different manufactures," said Prof Saul Faust, the director of the NIHR Southampton Clinical Research Facility, who will run the trial.
He added: "We just don't know how each of these vaccines is going to behave and we can't be certain vaccine supply will be efficient and secure from one manufacturer."
The trial has started the job of recruiting 6,000 people in the UK. Other countries will join the effort to bring the total up to 30,000.
Half of the volunteers will be given two doses of the vaccine around two months apart.
Janssen already has one large scale trial of its vaccine in which volunteers get one dose. This trial will see if two gives a stronger and longer lasting immunity.
It could take six to nine months before the results are available.
Health Correspondent Laura Foster explains what the latest Covid-19 vaccine news means
Hopes for the Janssen vaccine have been buoyed by Pfizer's preliminary data as they both target a part of the virus called the spike protein.
The seemingly successful jab injected part of the virus' genetic code into volunteers.
The Janssen vaccine instead uses a common cold virus that has been genetically modified to make it harmless and to look more like coronavirus at a molecular level. This should train the immune system to recognise and fight coronavirus.
This approach is similar to the vaccine designed by the University of Oxford and AstraZeneca, which is also being trialled in the UK. The subtle difference is the Janssen vaccine uses a virus that normally infects people and the Oxford group are using one that infects chimpanzees.
But all these approaches are relatively new and experimental. The Novavax jab, which uses the more traditional method of injecting viral proteins to train the body, started in September in the UK.

SOCIAL DISTANCING: Can I give my friends a hug?
TESTING: How do I get a virus test?
GLOBAL SPREAD: How many worldwide cases are there?

In total 25,000 people are already taking part in Covid trials in the UK.
The UK government has already put in advanced orders for six Covid vaccines, including 30 million doses of the Janssen jab.
Kate Bingham, the chairwoman of the UK's Vaccine Taskforce, said: "Many vaccines are needed both here in the UK, and globally, to ensure we can provide a safe and effective vaccine for the whole population.
"That is why the launch of this trial to establish the safety, effectiveness, and very importantly the durability, of the Janssen vaccine is so significant, and I would continue to encourage people to sign up and take part in vaccine trials."
Are you involved in the new Janssen vaccine trial? Get in touch by emailing haveyoursay@bbc.co.uk, external.
Please include a contact number if you are willing to speak to a BBC journalist. You can also get in touch in the following ways:
WhatsApp: +44 7756 165803
Tweet: @BBC_HaveYourSay, external
Please read our terms & conditions and privacy policy
If you are reading this page and can't see the form you will need to visit the mobile version of the BBC website to submit your question or comment or you can email us at HaveYourSay@bbc.co.uk, external. Please include your name, age and location with any submission.
- Published9 November 2020
- Published28 May 2021
- Published20 January 2021
