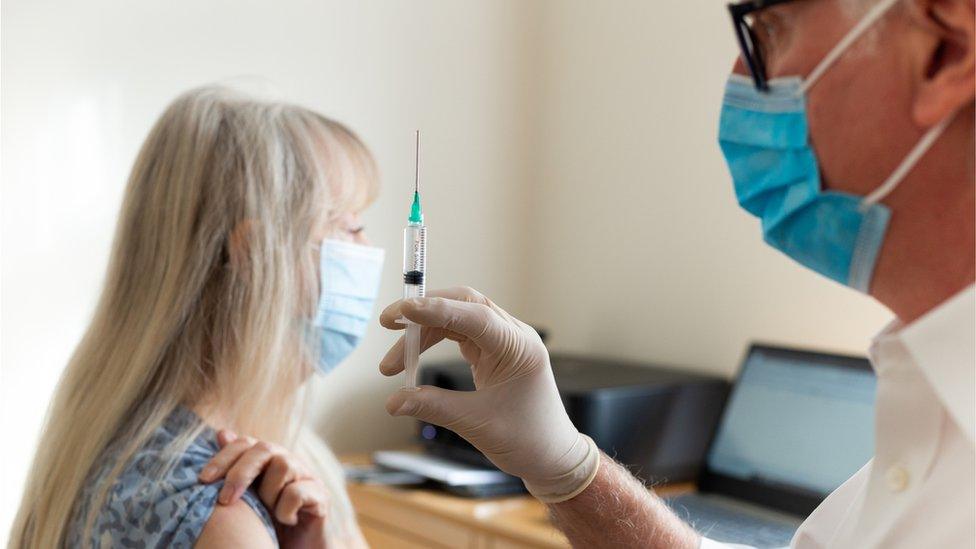
Covid: Pfizer and AstraZeneca approved as booster vaccines
- Published
- comments
The UK medicines regulator has approved the use of Pfizer and AstraZeneca as Covid booster vaccines, paving the way for a rollout ahead of the winter.
But the Joint Committee on Vaccination and Immunisation (JCVI), the UK vaccine advisory body, has not decided if they are needed, and who should be eligible.
The JCVI has said a third dose should be offered to people with severely weakened immune systems.
Up to half a million people over the age of 12 in the UK are in this group.
A separate booster programme against Covid-19 would aim to extend protection for millions more at high risk from the virus, although there is disagreement over whether this is really necessary.
AstraZeneca bosses have warned against rushing into offering boosters when the data showing it's needed after two doses isn't yet clear.
If needed, boosters could be given to frontline health and care staff, care home residents and over-70s first.
Earlier this week, ministers said the NHS was ready to go if booster jabs were given the green light.
Dr June Raine, chief executive of the Medicines and Healthcare products Regulatory Agency (MHRA), said: "I am pleased to confirm that the Covid-19 vaccines made by Pfizer and AstraZeneca can be used as safe and effective booster doses.
"This is an important regulatory change, as it gives further options for the vaccination programme, which has saved thousands of lives so far."
Scientists have been studying whether a mix of vaccines could provide better protection than three doses of the same jab. The data from these trials are likely to feed into any decision from the JCVI, who met today to discuss the findings.
- Published1 September 2021
- Published8 September 2021