Paxlovid: UK medicines regulator approves second Covid antiviral pill
- Published
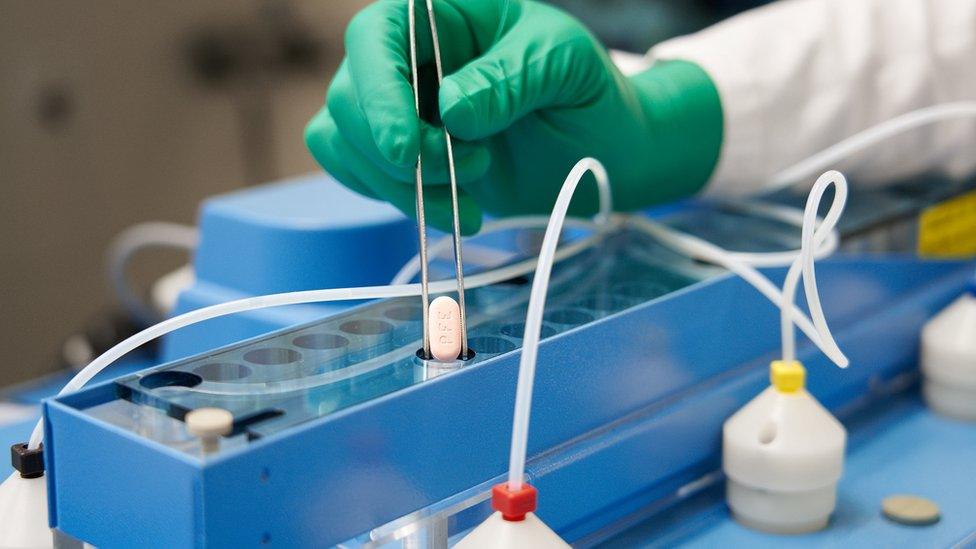
A promising new antiviral pill to treat symptomatic Covid has been approved by the UK medicines regulator.
The drug - Paxlovid - is intended for use soon after symptoms develop in people at high risk of severe disease.
In clinical trials it reduced the risk of hospitalisation or death by 89% in vulnerable adults.
The UK has ordered 2.75 million courses of the tablet which is partly based on an existing HIV medication developed by the US company Pfizer.
Paxlovid is the second new antiviral pill to be approved for Covid in the UK after a rival drug, molnupiravir, was given the green light by regulators in November.
The health secretary Sajid Javid said new antiviral drugs plus booster vaccines and increased testing mean the UK is in "the strongest possible position" to deal with the threat posed by Omicron in 2022.
Protease inhibitor
Paxlovid, known as a protease inhibitor, is designed to block an enzyme the virus needs in order to multiply. When taken alongside a low dose of another antiviral pill called ritonavir, it stays in the body for longer.
It has been approved for use in the UK in patients over the age of 18 years old who have a mild to moderate Covid infection but are at high risk of their illness worsening.
Interim data from clinical trials in 1,219 vulnerable patients who caught the virus found that 0.8% of those given Paxlovid were later hospitalised, compared with 7% of patients who were given a placebo or dummy pill.
The results were published in a press release and have not yet been peer reviewed by other scientists.
Dr June Raine, the chief executive of the UK Medicines and Healthcare products Regulatory Agency (MHRA), said: "We now have a further antiviral medicine for the treatment of Covid that can be taken by mouth rather than administered intravenously.
"This means it can be administered outside a hospital setting, before Covid has progressed to a severe stage."
The regulator said Paxlovid is most effective when taken in the early stages of infection and has recommended the drug be used within five days of the onset of symptoms.
Doctors say getting new antiviral pills quickly to vulnerable adults who test positive will be crucial if the treatments are going to have a major impact on hospitalisation and death rates.
Paxlovid will be made available through 70 new hospital clinics known as NHS Covid medicine delivery units, and sent directly to the door of eligible patients infected. Some of those most at risk of Covid have already been sent PCR tests to store at home and take immediately if any symptoms develop in an attempt to speed up the treatment process.

Paxlovid (pictured) is the second antiviral tablet to be approved in the UK for Covid.
Molnupiravir, another antiviral pill, is already being offered to the most vulnerable adults in the UK - including cancer patients and transplant recipients. It is also being tested on a wider pool of 10,000 people over 50 years old as part of a major study led by Oxford University which is now likely to be extended to include Paxlovid.
Earlier this month the UK increased its order for molnupiravir, made by the US drugs company Merck Sharp & Dohme, to a total of 2.3 million courses. On the same day, the French health authorities cancelled their entire order after what they described as disappointing clinical trial data.
A third new treatment for the disease, sotrovimab, has recently been approved and is being offered as an infusion in clinics and to hospital outpatients.
Related topics
- Published5 November 2021

- Published4 November 2021
- Published16 June 2021

- Published6 October 2021

- Published5 July 2022
