Blood tests may spare cancer patients chemo
- Published

Ben Cooke avoided chemo thanks to the blood tests
A blood test which can detect traces of cancer cells could spare thousands of patients unnecessary chemotherapy every year.
A major bowel cancer trial is examining whether the test can show if surgery has removed all of the tumour.
Doctors say half of patients with stage 3 bowel cancer are cured by surgery alone so by using chemotherapy they are over-treating many people.
About 1,600 bowel cancer patients are being recruited to the UK study.

Ben Cooke runs a hair salon on the King's Road in Chelsea, London, and also works as a stylist for fashion shoots.
In early March last year, he noticed some dark blood in his poo. He rang NHS 111 and was sent to A&E. He was diagnosed with stage 3 bowel cancer, which was successfully treated with surgery.
The gold standard treatment is to then have intravenous chemotherapy to mop up any remaining tumour cells and reduce the risk of the cancer returning.
But the chemotherapy used in bowel cancer, oxaliplatin, can cause painful tingling and numbness in the hands and feet, called peripheral neuropathy.
This nerve damage can be long-term, and Ben was worried it might affect his ability to do the job he loves.
"I would not be able to cope with that," he says. "I need to work - it's my therapy."
The 52-year-old enrolled in a study at London's Royal Marsden Hospital, which is evaluating whether a blood test can show if chemo is really needed.
His test showed he was clear of cancer, so he avoided intravenous chemotherapy.
Instead, like everyone taking part in the trial, he took an oral chemo tablet twice a day. This had minimal side effects and allowed him to carry on working.
"The fact that I didn't have any tingling in my hands has just been an absolute blessing," he says.
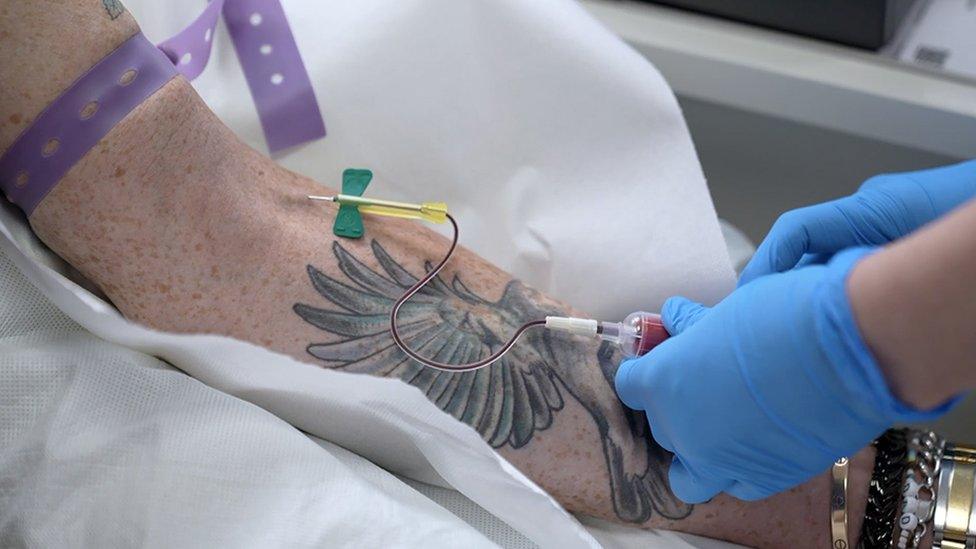
Ben's blood is extracted for the liquid biopsy
The blood tests work by looking for microscopic traces of cancer in the bloodstream called circulating tumour DNA. The presence of these markers indicates whether the patient has been cured by their surgery or not. Such tiny fragments would be invisible on a scan.
Ben's consultant at the Marsden, Dr Naureen Starling, is the principal investigator on the trial. She says the outcome could affect the way thousands of bowel cancer patients are treated every year.
"Half of patients with stage 3 bowel cancer are cured by surgery alone, so we are over-treating a large proportion of patients," she says.
The hope is that this specialised technology could spare many cancer patients unnecessary chemotherapy.
"That's good for the patient, it's good for the health service, it's good for cost savings within the NHS. That would be a win-win," says Dr Starling.
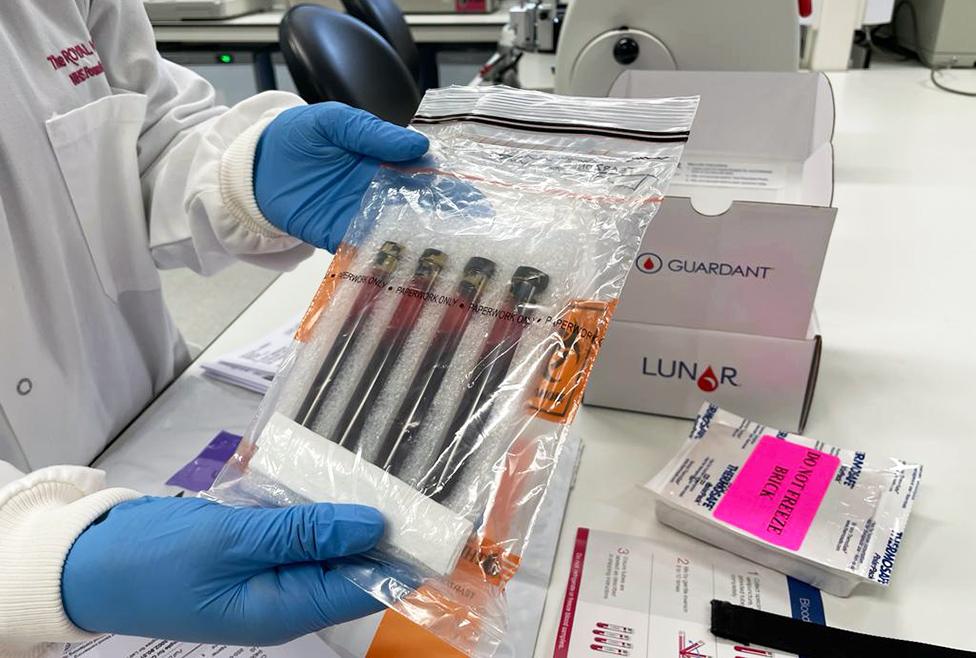
The liquid biopsies being packaged to send to California
The trial, called TRACC, external, is using a test created by US company Guardant Health. The samples are sent to their labs in California for analysis with the results coming back within around two weeks.
Survival outcomes
The trial, funded by the National Institute for Health & Care Research (NIHR) and Medical Research Council (MRC), will examine any difference in survival rates after three years between those patients whose treatment was guided by the blood test compared with the standard-of-care chemotherapy group.
Trials are also under way in the UK to monitor patients with lung and breast cancer in the same way.
Dr Starling says the potential for this new technology across cancer care is "immense", not just when it comes to detecting residual disease after surgery, but also for early diagnosis.
What is clear already, from multiple studies, is that so-called "liquid biopsy" blood tests can reveal the lingering presence of cancer long before it would be found using traditional methods.
A trial in Greece published in Nature in January, external, found that liquid biopsies could show cancer recurrence at least four years before it would be detectable via a scan. That study followed a small group of breast cancer patients after surgery.
At the American Society of Clinical Oncology conference in Chicago last June, a study in 455 bowel cancer patients, external found that by using the blood tests to guide treatment, the number of patients needing post-surgery chemotherapy was nearly halved without the risk of relapse.
But Dr Starling says the far bigger randomised trial in the UK is essential to calibrate exactly how much reliance can be placed on liquid biopsies, especially when it means considering the withdrawal of chemotherapy.
The tests have already been available to private patients.
Susanne Winter, an artist from Surrey, was diagnosed with stage 3 bowel cancer in March 2022 and had successful surgery to remove the tumour and some cancerous lymph nodes.
She initially thought she would need chemotherapy to ensure the cancer was entirely gone, but she had the ctDNA test done privately which showed she was clear of cancer.
Susanne, 58, had cancelled all her commitments to prepare for several months of chemotherapy, but the negative test result meant she was free to concentrate on her art. She even had two works accepted for the Royal Academy summer exhibition.
She feels incredibly lucky to have avoided chemotherapy. "I knew how toxic it can be. You're psyching yourself up for it, so to hear that you aren't going to need it is just unbelievable," she says.
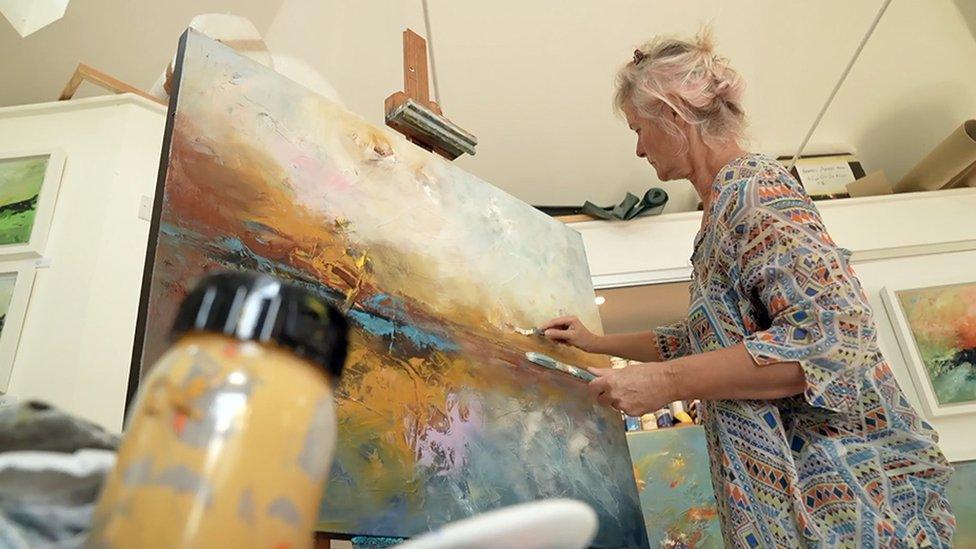
Susanne Winter was able to carry on painting
What is considered the holy grail of cancer detection is to be able to spot the disease at the very earliest stage, when it is most easy to cure.
Blood tests are also being trialled to see if they can diagnose a whole range of cancers.
More than 140,000 volunteers aged 50-77 have been recruited across England to see if the test could pick up more than 50 types of tumours, most of which have no screening programmes.
The NHS-Galleri trial is made by Californian company Grail, external, and some interim results are due early next year.