NHS not ready for new Alzheimer's drugs lecanemab and donanemab, warns charity
- Published
Alzheimer's patients could lose out on two groundbreaking new drugs because the NHS is unprepared, a leading charity has told BBC Panorama.
Lecanemab and donanemab slow down the early stages of the disease - which is the most common form of dementia.
But Alzheimer's Research UK says the NHS is not ready to roll out the drugs, which could be licensed this year.
The treatments would then be subject to an assessment of cost and benefits before they are made available.
Lecanemab and donanemab represent a step forward because they target one of the causes of Alzheimer's, rather than treating the symptoms.
However, their effectiveness depends on early diagnosis - and very few people have the specialist scans or investigations which would be needed.
Questions also remain over potentially harmful side-effects of the drugs and whether the benefit they offer represents value for NHS money.
Dawn, who's 62 and from Hampshire, is on a new donanemab trial, aimed at studying optimum dosing levels.
She started noticing memory problems last year, forgetting recent events and sometimes struggling to follow a television programme.
"I just knew something wasn't right," she says.
Dawn receives her donanemab treatment through IV infusion
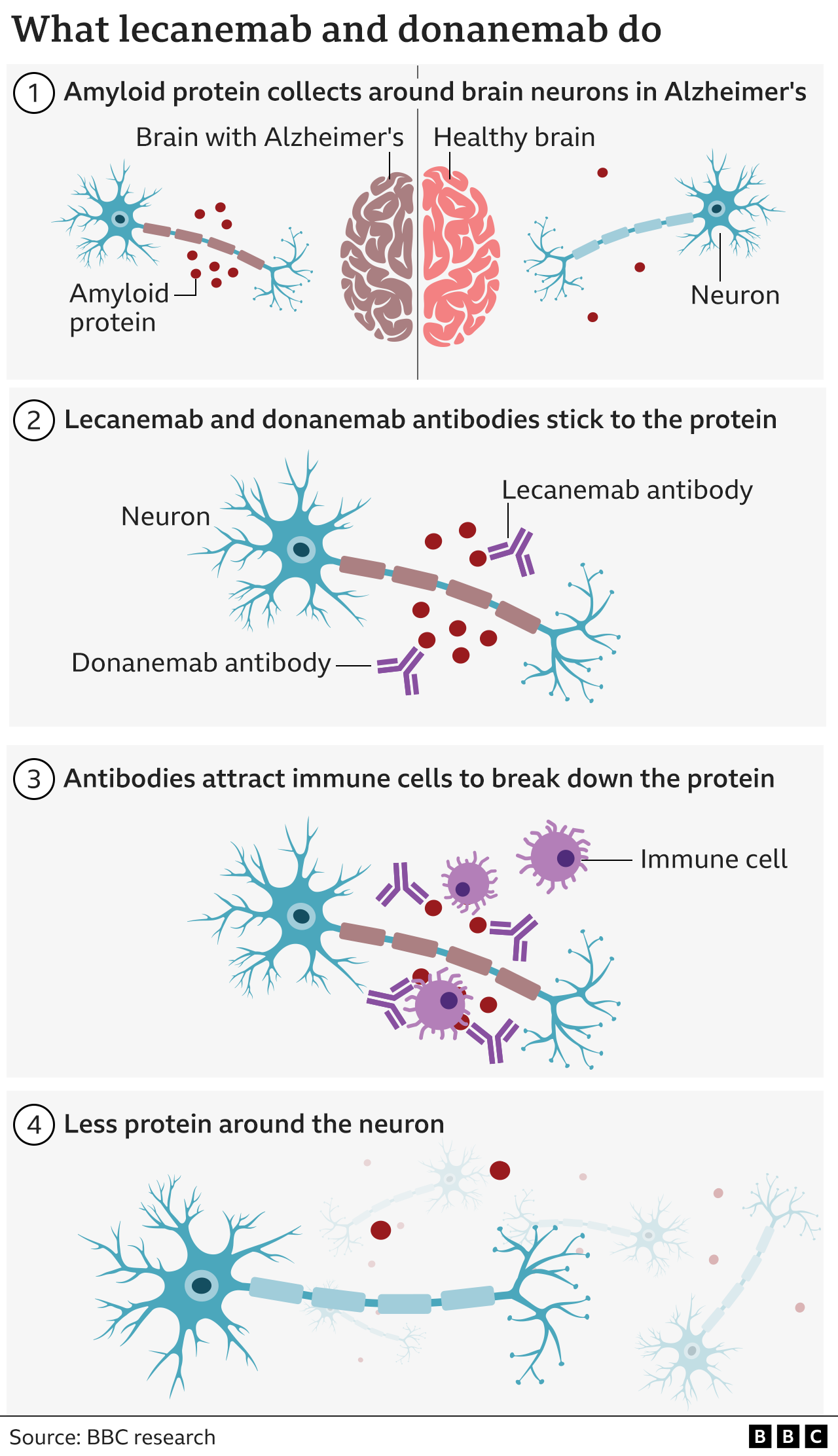
Tests revealed raised levels of a rogue protein in her brain called amyloid - a key marker of Alzheimer's disease.
Amyloid forms clumps or plaques around neurons, which are cells in the brain that transmit information and instructions throughout the body.
This process can start up to 20 years before the damage to brain cells becomes apparent and the first symptoms of dementia emerge, such as memory loss, or confusion.
Donanemab and lecanemab are antibodies, like those made by our body to attack viruses or bacteria. They have both been engineered to bind to amyloid and help our immune system clear it from the brain.
Dawn is hopeful the drug will help her: "If it slows it down, then I'll be able to function as I'd like to and do some of the things I'd still like to do."

Fergus Walsh follows patients with Alzheimer's disease, who have been taking two new drugs that have been shown to slow down its progression. Is this a turning point in its treatment?
Watch on BBC One on 12 February at 20:00 (20:30 in Northern Ireland and 22:40 in Wales) or on BBC iPlayer now (UK Only)

In global trials involving hundreds of early-stage Alzheimer's patients, the drugs were shown to slow cognitive decline by between about a quarter and a third over 18 months.
Consultant neurologist Dr Cath Mummery, who is head of clinical trials at the Dementia Research Centre, University College London, says this would make a meaningful, if small, difference to individual patients: "Over 18 months, that gives you about five months at a higher function."
Lecanemab - produced by Eisai and Biogen - is already licensed in the US, external where it costs about £20,000 per patient per year.
The UK medicines regulator - the Medicines and Healthcare products Regulatory Agency (MHRA) - is assessing the drugs and is expected to approve lecanemab within the next few months, with the licence for donanemab - made by Eli Lilly - following slightly later.
However, both drugs come with potentially serious side effects, most notably swelling and bleeding in the brain. Three of the 853 people who took donanemab during the clinical trial, died as a result of the treatment.
As a result, patients need very careful monitoring via MRI brain scans.
The side effects of the drugs and their limited effectiveness has led some critics to dismiss them as a dead end, external.
Dr Cath Mummery disagrees.
She says, "For the first time, we've got drugs that show that you can alter the course of Alzheimer's disease, and that's an extraordinary thing."

Consultant neurologist Dr Cath Mummery: The new drugs offer a new approach to tackling Alzheimer's
Dr Mummery believes this may be the first move towards managing Alzheimer's as a chronic disease and keeping sufferers well and stable for as long as possible. Until now, she says, doctors could only give supportive and palliative care for what is, in effect, a terminal disease.
But even if the drugs are licensed in the UK, it does not mean they will be immediately available on the NHS.
The National Institute for Health and Care Excellence (NICE) and the Scottish Medicines Consortium will assess the effectiveness of lecanemab and donanemab, and whether they represent value for money.
A briefing paper for NHS England, external estimates that between 50,000 and 280,000 patients might be eligible for the new treatments if NICE recommends that the drugs are used by the health service. The cost of the drugs - and administering the treatment - would be between £500m and £1bn per year.
However, even if the drugs are approved, Alzheimer's charities say that the NHS is unprepared for their delivery.

Support information about dementia / Alzheimer's can be found through BBC Action Line.

To be eligible for either drug, patients would have to be in the early stages of Alzheimer's and have had a PET scan, external or lumbar puncture, external to confirm high levels of amyloid in their brain. Currently, only 2% of dementia patients receive either of these "gold standard" methods of diagnosis, according to Alzheimer's Research UK.
It is hoped that a blood test to detect amyloid levels, external may be available within five years. However, Dr Susan Kohlhaas, director of research at Alzheimer's Research UK, says that, currently, fewer than two thirds of sufferers receive any dementia diagnosis at all.
"Would we accept that for any other disease area, much less than for the biggest killer in the UK?" she asks. "This is a major issue that we need to start addressing now."
NHS England says that the pandemic had a "significant impact" on dementia diagnosis rates, but (as of November 2023) they are the highest they have been in three years. It adds that a dedicated programme team has been established "to accelerate NHS preparations for the rollout of any future Alzheimer's treatment".
Paul, 73, from North Yorkshire, first started noticing his loss of memory a couple of years ago and it has since got worse.
He was referred for a brain scan by his GP and then waited five months for an appointment with a memory clinic. He still does not have a formal diagnosis.
He says the delays have made him "really worried and upset" because his condition has deteriorated in recent months, and he is aware that the new drugs need to be given to those with only mild symptoms of Alzheimer's.
"I have a young family, so the longer I can be around the better."

Delays in formal diagnosis have left Paul "worried and upset"
For most people, advancing age is the biggest risk factor for Alzheimer's.
But in rare cases it is triggered by a faulty gene, meaning the onset of symptoms can happen in someone's 30s.
Pete, 33, from North Lincolnshire, lives under the shadow of Alzheimer's. His mother first developed symptoms of cognitive decline around his age and died from Alzheimer's aged 41.
A genetic test in 2018 confirmed that Pete had inherited a rare gene for early-onset Alzheimer's called presenilin 1.
It was passed down from his mother and grandmother, who also developed Alzheimer's in her 30s. With each generation there is a 50:50 chance that children will have inherited the gene.
Pete has no symptoms at present and is part of a clinical trial of two Alzheimer's drugs, external. Lecanemab is one of those, as it targets amyloid - while the other is E2814, which targets tau, another protein implicated in the development of the disease, which forms tangles inside neurons, harming their ability to communicate.

Pete hopes his treatment will delay the onset of Alzheimer's indefinitely
"If these drugs I'm on don't work I will get Alzheimer's," he says. "The age range varies, and if I'm lucky, onset won't happen until my mid-to-late 40s. But it will happen."
Lecanemab has so far been tested on patients who already have a large build-up of amyloid in their brain and are showing symptoms of decline.
The hope is the effect could be bigger and longer lasting in someone like Pete.
"I do my best to remain grounded and there are no guarantees, but I joked with someone the other day that I could be the first person to beat Alzheimer's."