Brazil halts trials of China's Sinovac vaccinepublished at 08:45 GMT 10 November 2020
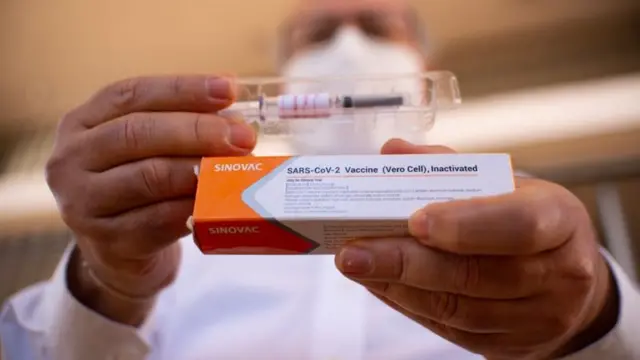 Image source, Getty Images
Image source, Getty ImagesBrazilian health authorities have suspended clinical trials of the Chinese Covid-19 vaccine, Coronavac, because of what they called a "severe adverse" incident.
Brazilian health regulator Anvisa said the incident happened on 29 October, but did not give further details. Butantan, the medical research institute conducting the trial, is to hold a news conference on Tuesday at 11:00 local time (14:00 GMT).
The Sinovac vaccine is one of several in final-stage testing globally. But China has already been using it to immunise thousands of people at home in an emergency programme. There was no immediate response from Sinovac.