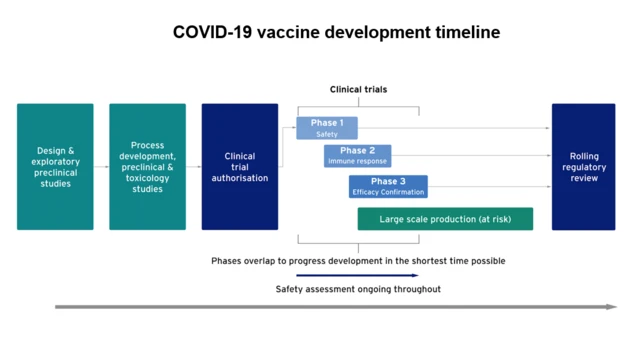
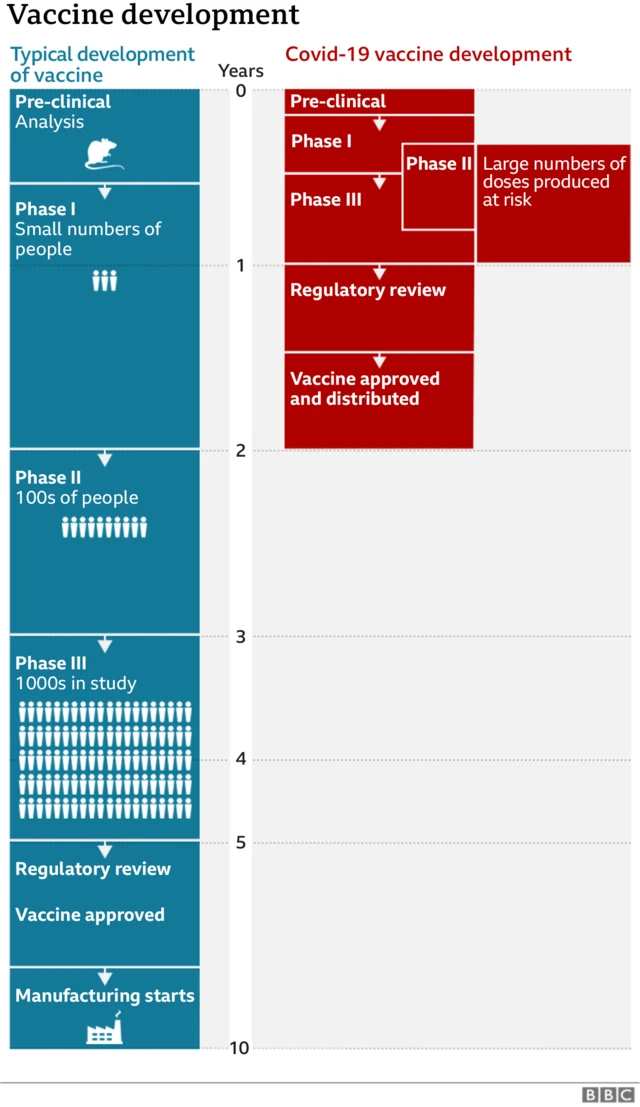
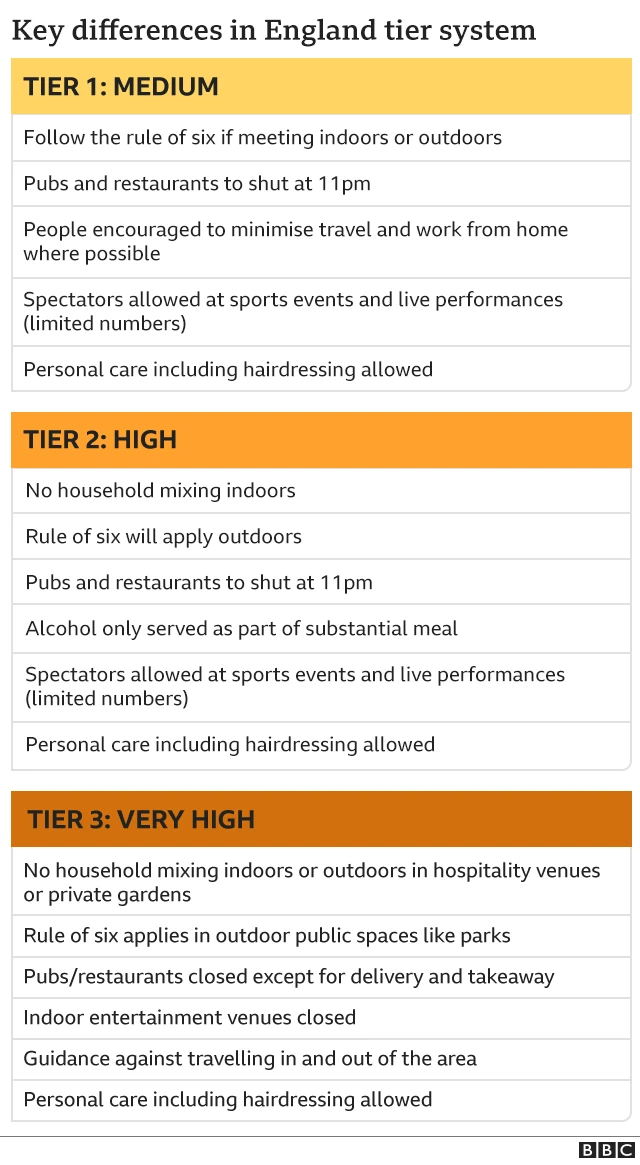
'Immunity seven days after second dose'published at 10:33 GMT 2 December 2020
Asked how the MHRA regulatory body has been so quick to approve the vaccine, Dr June Raine stresses its standards are equivalent to all international standards.
"The public can be absolutely confident the standards we have worked to are the equivalent of those around the world," she says.
How long is the lag before the vaccine becomes effective?
Prof Sir Munir Pirmohamed says people will be immune seven days after the second dose.
There will be partial immunity after the first dose, he says, explaining they have seen some protection occurring after day 12 of the first dose.