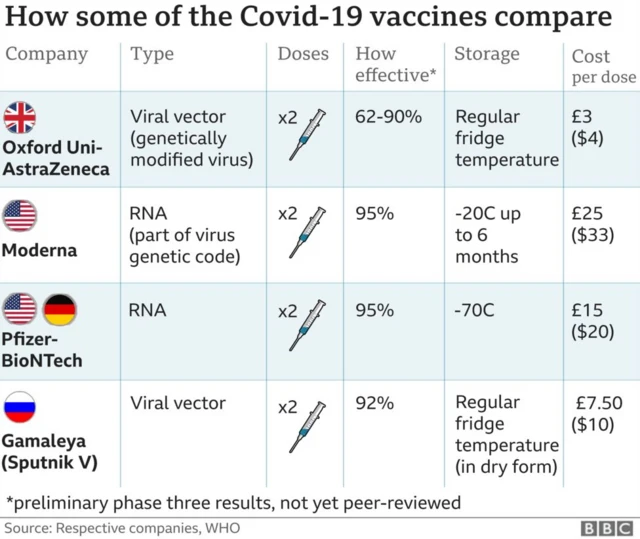
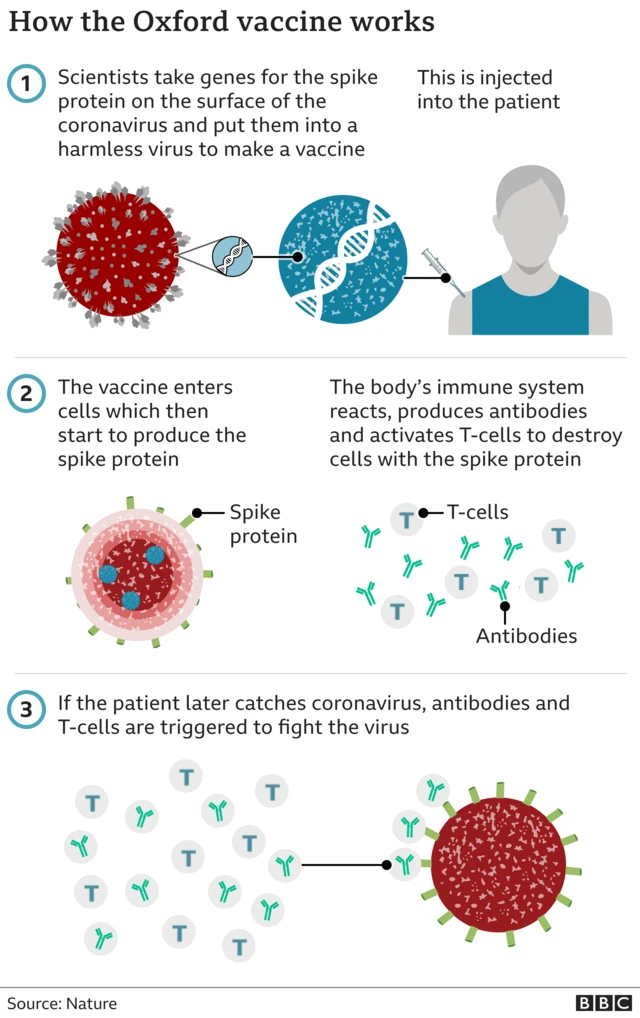
Pfizer vaccine 'can now be considered for pregnant women'published at 11:05 GMT 30 December 2020
Dr June Raine, chief executive of British regulator the MHRA, also gives some updates on its advice on the first vaccine that was approved in the UK – the Pfizer jab.
She says the MHRA now says the vaccine can now be considered for use by pregnant women and women who are breastfeeding when the potential benefits outweigh the risks.
Addressing people with allergies, she says there is growing evidence that the risk is minimal and anyone with allergy to food or any other vaccine can have it. However, she stresses that people with allergies to any of the ingredients in the Pfizer vaccine shouldn’t receive it.
She also says that the regulator is advising the second dose of the Pfizer vaccine comes at least 21 days after the first dose, rather than exactly 21 days after, allowing for a potentially longer interval between the two vaccines.