Lithium: A metal that floats on oil and powers our phones
- Published

Lithium, a key ingredient in lightweight batteries, is already powering the modern world, and could be key to getting the world to reduce its reliance on fossil fuels.
Look at a satellite image of South America. Halfway down on the left-hand-side is a distinctive white splodge.
Close up, that splodge turns out to be one of the most extraordinary and unspoilt places on earth, the world's biggest salt flat.
It is a crisp, perfectly flat white plain, like freshly fallen snow, 100km (60 miles) across and 3,600m (12,000ft) up in the remote Bolivian Andes.
This is the Salar de Uyuni and this hauntingly beautiful place could be part of the key to tackling climate change, helping to wean the world away from fossil fuels.
Which is why, pristine as it may be, the chances are that 50 years from now it will all be gone - dredged, crystallised and then carted away.
That's because under its thick salt crust, the Salar de Uyuni is also the world's biggest single deposit of lithium, accounting for perhaps a third of the world's resources of this alkaline metal.
Go back to the 1980s, and lithium was one of the more obscure members of the periodic table, much as its next-door neighbour beryllium remains today.
That all began to change in 1991, when Sony launched the first ever portable gadget powered by a lithium-ion battery.
Today, of course, the words "lithium" and "battery" are almost synonymous - this soft metal is in all our smartphones, tablets and laptops.
The secret of lithium's success is that it is the third element of the periodic table, after the gases hydrogen and helium.
Its simple atoms, containing just three protons each, make lithium the lightest of all metals.
In its pure form, lithium will actually float on the oil it is normally stored in by chemists.
And it is stored in oil, under the inert gas argon, for a reason.

For lithium is also the first of the alkali metals - like its near kin sodium and potassium, it will react spontaneously to water, though not quite as violently as those other two.
All of which makes lithium an ideal material for light-weight batteries.
"We think of batteries as producing an electrical current, sending electrons around a circuit," explains chemistry professor Andrea Sella of University College London.
"But of course, as a chemist, I am interested in what goes on inside the battery. And for every electron, a lithium ion also has to move inside the battery."
Being so light, the atoms slip easily between the layered materials that make up the battery.
And its lightness also makes lithium the most energy dense of battery materials - meaning it stores the most energy for a given weight.
This is why lithium is so important for the battle against climate change. It is the optimum battery material if you need to carry your energy store with you - in a gadget, or in a car.
Batteries are not the only things that take advantage of lithium's unique electrochemical properties.
The human body does as well.
"Lithium saved my life. I would be dead or in the back wards without it," says Kay Redfield Jamison, professor of psychiatry at Johns Hopkins School of Medicine.
Professor Jamison is an expert on bipolar disorder - also known as manic depression - who herself suffers from the condition.

Like many sufferers of bipolar illness she says pills made of purified lithium carbonate, the same naturally-occurring substance that is mined in many salt lakes today, has helped smooth out the frenetic highs and suicidal lows of this disease.
"When against medical advice I haven't been on it, I almost immediately started getting manic and then suicidally depressed," she says.
And while those on lithium do not exactly have a normal life - the pills can cause nausea and leave them feeling emotionally inhibited - it has prevented many from taking their own lives.
How this happens remains something of a mystery.
However, it probably has something to do with the fact that our nerves and brains don't operate using flows of electrons, as you may imagine.
Instead, they rely on flows of ions - positively charged particles of sodium and potassium.
It may be that lithium ions soften the swings between overactive and underactive flows of these ions that researchers theorise are behind bipolar disorder.
Medicine is likely to continue to use a steady supply of lithium, but according to the Chilean mining company SQM, the reason demand for the metal has been growing at 20% a year is increased demand for lithium batteries.
And the biggest driver of demand is not for gadgets, but for cars.
Fuel-efficient hybrid cars, which use electric motors powered by lithium batteries alongside conventional gasoline or diesel engines, are proving very popular.
The market for pure electric vehicles has been slower to develop, thanks in large part to the limitations of lithium batteries.
"In its pure form, lithium actually has the same energy density as gasoline," explains Prof Nigel Brandon of Imperial College in London, one of thousands of researchers engaged in a huge worldwide effort to eke out ever better performance from our batteries.
"But we cannot use lithium in its pure form. We have to store it in other materials, and that dilutes the energy density of batteries in practice."
Throw in the weight of the two electrodes, the casing, the electrolyte fluid and so on, and the energy-storing performance of your average electric car battery turns out to be only one 50th as good as a tank of petrol.
How a lithium-ion battery works
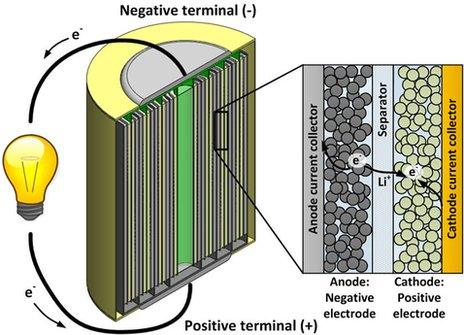
It is conventional with lithium batteries to refer to the negative electrode as the anode, and the positive electrode as the cathode. The two electrodes, with an electrically insulating separator between them, are often rolled up like a Swiss roll.
During discharge, electrical current flows from the anode to the cathode through the device the battery is powering (symbolised here by a light bulb). Simultaneously, positively charged lithium ions travel from the anode to the cathode through the separator.
On reaching the cathode, the lithium ions embed themselves in its metal oxide structure, which simultaneously accepts electrons from the external circuit.
The anode is typically made of carbon, the cathode is typically made of a cobalt or manganese oxide. The electrolyte (the liquid surrounding the electrodes) is usually composed of lithium salts in an organic solvent, such as ether.
During charging the process occurs in reverse.

That matters, because the less energy the battery can store, the more limited the car's range.
The limited range of electric cars is one of the main reasons people are unwilling to switch to them.
But the good news is there is scope for improvement.
Professor Brandon reckons we could see a five-fold increase in energy density over the next two decades, perhaps even 10-fold or more, if new technologies prove successful.
A lot of current research is focused on "lithium-air" batteries, where much of the battery would be replaced with oxygen drawn from the atmosphere.
Even so, says Professor Brandon, the limits of lithium battery chemistry mean they will never come near gasoline in terms of energy density.
Indeed, in most handheld gadgets improvements in running time have had less to do with the performance of batteries than with the great steps that have been made reducing power consumption.
The same is likely to be true for electric vehicles for the foreseeable future - engineers will have to design them around the limitations of batteries.
Limits on energy density are not the only problem, because it is not the only thing you care about in a battery.
Otherwise, why have lead-acid batteries in traditional petrol-driven cars, given that lead is the heaviest stable element in the periodic table?
The reason is that lead provides two things that lithium currently cannot - the surge of power needed to fire up your engine, and incredible durability over thousands of cycles of the battery, no matter how hot or cold the weather.
As an alkali metal, lithium's high reactivity turns out to be a bit of an Achilles' heel, because unwanted chemical reactions inside the battery cause it to degrade over time.
And while that may be fine in a phone, that has a typical working life of two-to-three years, it is much more of a headache if you want your car battery to last closer to a decade.
Durability is not the only trade-off that researchers like Prof Brandon have to grapple with.
The battery also has to be safe, and cheap.
And, as certain plane and car manufacturers have discovered, lithium batteries do occasionally overheat and catch fire.
Nonetheless, we prize the freedoms batteries give us and the electro-chemical properties of lithium mean it will remain central to their future.
All of which brings us back to South America.
Bolivia's Salar de Uyuni is one corner of a "Lithium Triangle" that also takes in the northern ends of Chile and Argentina.

These three countries dominate world lithium supplies thanks to the incredible geological forces shaped the South American continent.
The subduction of the Pacific plate under Chile's coast, and the resulting tectonic uplift of South America, created large localised depressions which cause water to flow into lakes instead of escaping into the sea.
The lithium salts that dissolve out of the surrounding rocks collect in these great lakes.
And the Andes themselves play a key role.
They squeeze almost every drop of water out of the prevailing winds off the Pacific, making the western slopes some of the most arid spots on earth.
This dry climate causes these lakes to evaporate, leaving behind the crystallised salts you see at the salt flats of the Salar de Uyuni and at the Salar de Atacama, in the middle of the Atacama desert - the driest place on earth.
The Salar de Atacama is not as picturesque as the Salar de Uyuni because of the dust that blows in from the surrounding desert, but it is the biggest single source of lithium currently being mined.
So why is the Salar de Uyuni virtually untouched while this place is so busy?
German Perez, head of mining production at SQM's Salar de Atacama plant in Chile, explains the extraction process
Geography is part of the reason.
The Atacama deposit is richer in lithium than Uyuni and is easier to exploit because it is nearer the sea and, instead of stuck at the top of a mountain range, it is on a flat plain.
That makes the roads and infrastructure needed for export much cheaper.
But politics is also a key factor.
The Salar de Atacama is controlled by a government in Santiago that has a long and happy working relationship with the foreign mining companies who have exploited Chile's largest mineral resource, copper.
Contrast that with the radical-left Bolivian government which has vowed not to sell out to Western companies - assuming those Western companies would trust the government not to expropriate them.
But, if the world is to meet the future demand, other deposits will need to be opened up.
Most are in problematic locations - Tibet, Afghanistan, and of course Bolivia.
If the Bolivian government can learn to work with foreigners who have the necessary expertise and deep pockets to bring the stuff to market, then the Salar de Uyuni could prove a bonanza for one of the poorest countries in South America.
And the Bolivians have just begun a pilot mining project.
So visit this incredible location now, if you can, because there may not be much left of it once the lithium miners have finished their work.
Listen to the latest from Business Daily or browse the podcast archive
Follow @BBCNewsMagazine, external on Twitter and on Facebook, external
- Published8 November 2013

- Published18 November 2013
- Published23 November 2013
