Covid-19: Novavax vaccine shows 89% efficacy in UK trials
- Published

Biotech firm Novavax has labs in the US (pictured) and has carried out trials in the UK and South Africa
A new coronavirus vaccine has been shown to be 89% effective in large-scale UK trials.
The Novavax jab is the first to show in trials that it is effective against the new virus variant found in the UK, the BBC's medical editor Fergus Walsh said.
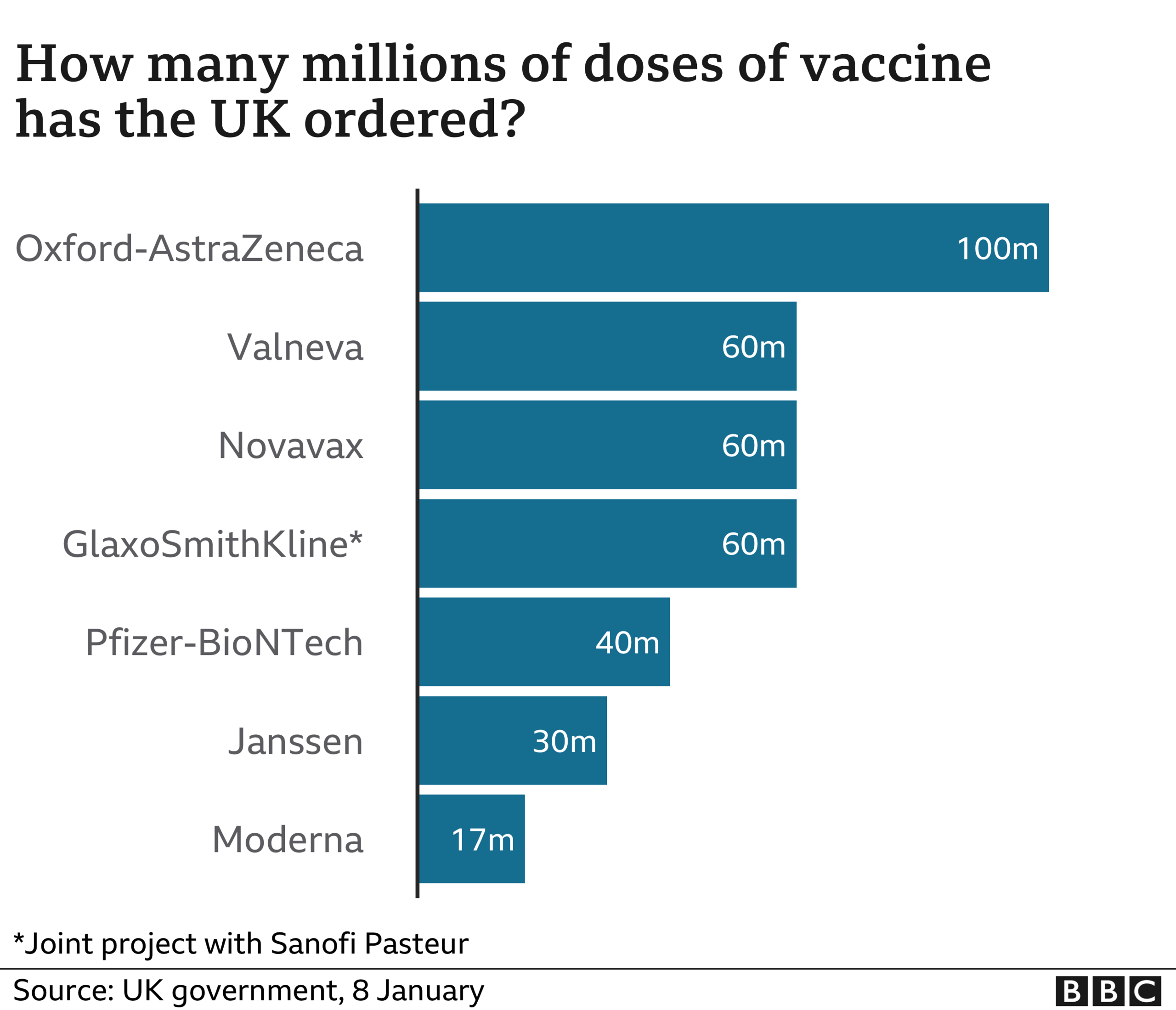
The UK has secured 60 million doses of the jab, which will be made in Stockton-on-Tees in north-east England.
Meanwhile, a single-dose vaccine developed by Janssen is 66% effective, trial results have shown.
Janssen, a company owned by Johnson & Johnson, is also investigating whether giving two doses will give either stronger or longer-lasting protection.
The company said its initial findings showed one dose prevented 85% of severe cases.
Both the Novavax and Janssen jabs will need to be reviewed by regulators before they can be used.
The PM welcomed the "good news", with doses of the Novavax jab expected to be delivered in the second half of this year if approved for use by the Medicines and Healthcare Products Regulatory Agency (MHRA), the government said.
The UK has so far approved three coronavirus vaccines for emergency use - one from Oxford University and AstraZeneca, another by Pfizer and BioNTech, and a third from drug firm Moderna.
The Novavax jab, which is given in two doses, was shown to be 89.3% effective at preventing Covid-19 in participants in its Phase 3 clinical trial in the UK, and around 86% effective at protecting against the new UK variant. The jab's efficacy against the original Covid-19 strain was calculated to be 95.6%, external.
The Phase 3 trials - the final stage before a vaccine is looked at by a regulator - enrolled more than 15,000 people aged between 18-84, of whom 27% were older than 65, US firm Novavax said.
In the South African part of the trial, where most of the cases were the South African variant of the virus, the vaccine was 60% effective among those without HIV.
Stan Erck, chief executive of Novavax, said the results from the UK trial were "spectacular" and "as good as we could have hoped", while the efficacy in South Africa was "above people's expectations".
He told the BBC the manufacturing plant in Stockton-on-Tees should be up and running by March or April, with the company hoping to get approval for the vaccine from the MHRA around the same time.
Novavax chief executive: UK trial results are "spectacular"
Minister Lucy Frazer told BBC Breakfast the government could not put an exact timeframe on when the Novavax jab might be approved as the regulation process is "out of our control".
Health Secretary Matt Hancock said the new vaccine would be "another weapon in our arsenal to beat this awful virus", if approved.
Prof Paul Heath, chief investigator of the UK Novavax trial, said the trial findings were "enormously exciting", particularly because of the jab's efficacy against the UK variant.


These extremely encouraging trial results suggest another powerful vaccine against coronavirus could soon be within reach.
It works in a slightly different way to the ones that are already available - but does the same job of teaching the body's immune system to recognise and fight the pandemic virus.
What is more, it appears to be effective against emerging and more infectious variants of coronavirus too - something scientists have feared might not be possible because the vaccines were all designed to match the original virus, not these new, mutated versions.
Protection against illness from the new UK variant was high - around 86%.
Even with the South Africa variant, which has undergone the most worrisome changes, it offered a level of protection similar to that given by flu shots against influenza.

England's chief medical officer Prof Chris Whitty said, external, if the jab is approved, it "increases our future resilience" against the virus.
Nadhim Zahawi, the UK minister responsible for the vaccine rollout, said he was "particularly thrilled" to see the positive results as he had taken part in Novavax's trials himself.
Covid vaccine safety: How does a vaccine get approved?
In total, the UK has ordered 100 million doses of the Oxford-AstraZeneca vaccine and 40 million of the Pfizer-BioNTech vaccine - both are currently being rolled out in the UK.
Another 17 million doses of the Moderna vaccine, which was approved by the MHRA in early January, are expected in the spring.
The aim is to give everyone in the top four priority groups - up to 15 million people - a first dose by mid-February.
Pfizer and Moderna vaccines rely on technology that has not been used in previous vaccines, but the Novavax jab uses a more traditional method of recreating part of the spike protein of the virus to stimulate the immune system.
Like the Oxford vaccine, the Novavax jab can be stored at regular fridge temperature - which means it can be distributed more easily.

More than 7.4 million people in the UK have so far received a first dose of a coronavirus vaccine, according to the latest government figures., external
Meanwhile, the latest estimate for the UK's R rate, external from the government's scientific advisory group, Sage, is 0.7 to 1.1. It means that on average, every 10 people with the virus will infect between seven and 11 other people. Last week, the R rate was between 0.8 and 1.
The latest growth rate range is between -5% and 0%, indicating that the number of new infections is broadly flat or shrinking by up to 5% every day.

TESTING: How do I get a virus test?
LOOK-UP TOOL: How many cases in your area?
GLOBAL SPREAD: How many worldwide cases are there?

Separately, the EU's vaccine contract with AstraZeneca has been published, in a growing row over reduced supplies of their jab.
Kate Bingham, who used to chair the UK's Vaccine Taskforce, said the reason the UK had a good supply of vaccines compared with other countries was because of its ability to get clinical trials completed quickly and at a high standard through the NHS's registry - with some 400,000 volunteers signing up "before the US even started their Phase 3 studies".
The UK recorded a further 1,239 deaths within 28 days of a positive coronavirus test on Thursday. There have also been another 28,680 new infections.


LOCKDOWN LEARNING: Need some assistance with home-schooling? BBC iPlayer is here to help
THE BIG SHORT: Did this film inspire the GameStop stock market "phenomenon"?

How have you been affected by the issues relating to coronavirus? Email haveyoursay@bbc.co.uk, external.
Please include a contact number if you are willing to speak to a BBC journalist. You can also get in touch in the following ways:
WhatsApp: +44 7756 165803
Tweet: @BBC_HaveYourSay, external
Please read our terms & conditions and privacy policy
If you are reading this page and can't see the form you will need to visit the mobile version of the BBC website to submit your question or comment or you can email us at HaveYourSay@bbc.co.uk, external. Please include your name, age and location with any submission.
Related topics
- Published29 January 2021
- Published29 January 2021
- Published29 January 2021

- Published2 April
- Published28 January 2021
- Published28 January 2021
