Coronavirus: Virus provides leaps in scientific understanding
- Published

A worker takes away an escaped giant salamander from a seafood market, which was shut down due to its connection to the spread of the coronavirus, in Wuhan, January 2020
In January 2020, two scientists published the entire genetic code of a coronavirus that was soon to wreak havoc around the world. It marked the start of a year of intense and rapid scientific endeavour, to work out how we might fight the virus.
Eddie Holmes had the genetic blueprint for the coronavirus in his possession for exactly 52 minutes before he put it online.
Prof Holmes is based at the University of Sydney, where he works on the emergence of infectious disease - an area of research that was suddenly thrust into the spotlight at the beginning of 2020. He has worked closely, for several years, with Prof Yong-Zhen Zhang, who was at the Chinese Centre for Disease Control in Beijing.
Prof Zhang sequenced the genome of the virus that closed down the world.

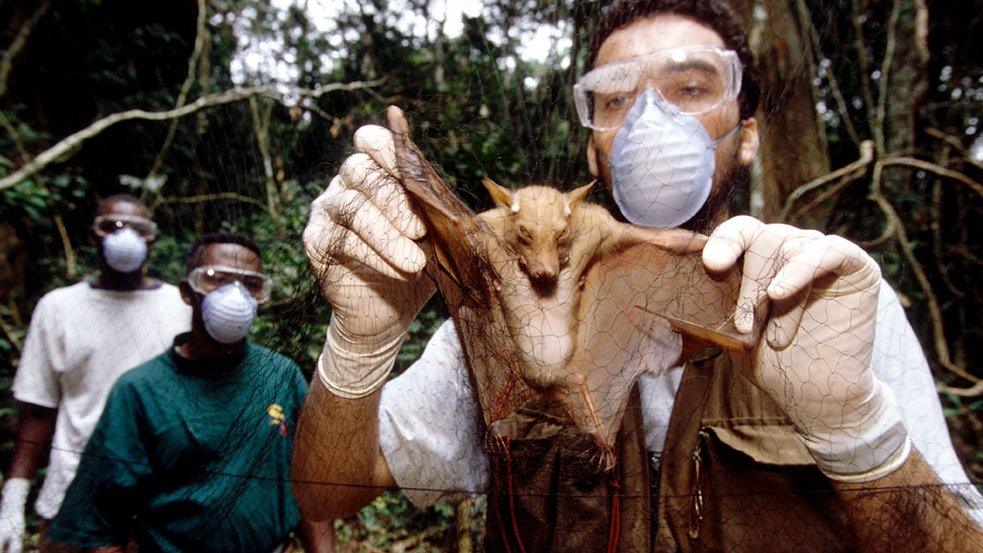
Prof Zhang and Prof Holmes examine a rat in a trap during an infectious disease research trip to China in 2013
He collected samples taken from some of the first patients in Wuhan Central Hospital, where a cluster of mysterious pneumonia cases had emerged. Many of those patients had a link to a seafood and wildlife market in Wuhan.
When he examined the code, Prof Zhang immediately saw that this was a coronavirus. It looked very similar to Sars - the respiratory disease that caused a deadly outbreak in Asia in 2002.
"That was on 5 January," recalls Prof Holmes. "And we just thought,oh no. It's Sars back again."
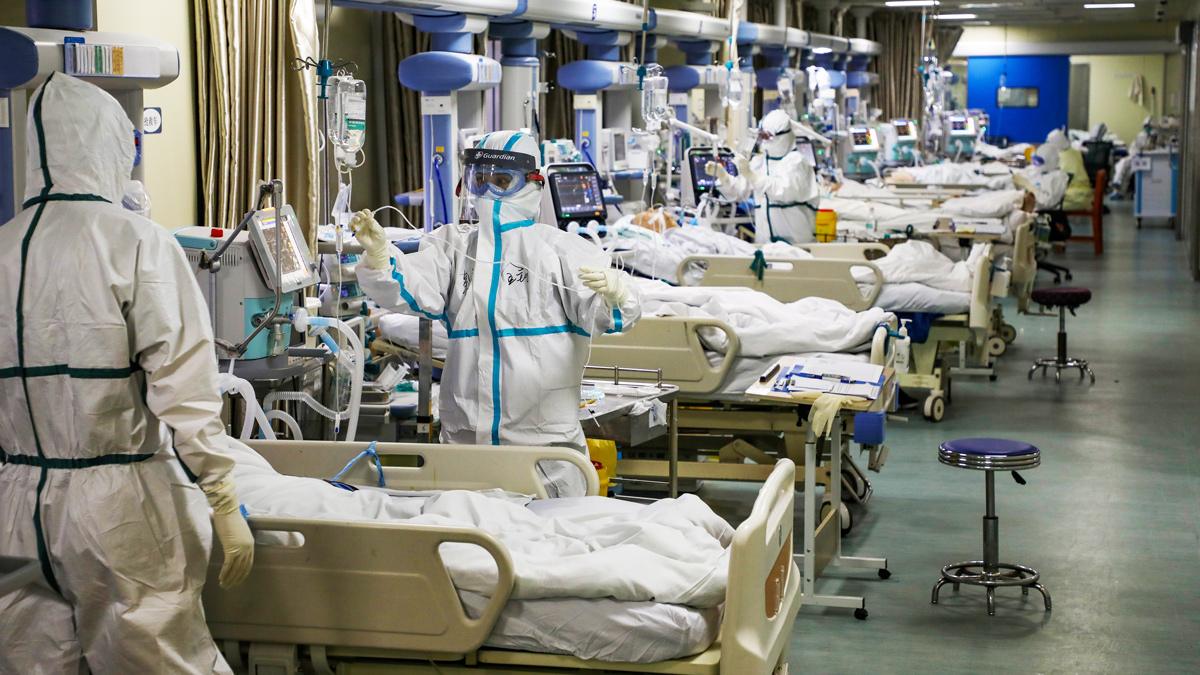
Medical workers in protective suits with Covid-19 patients in Wuhan, China, February 2020
But this code - this virus - was different. It was new. Prof Zhang and Prof Holmes quickly submitted a paper describing what they had seen and, as the week wore on, a buzz of public health speculation about what the novel virus might be started on social media.
"I didn't sleep," Prof Holmes tells me. "It was weighing on my conscience." In Sydney, it was early on 11 January when Prof Holmes phoned his colleague in China and asked his permission to publish the sequence. "Zhang was on a plane, strapped into his seat," Prof Holmes recalled. "He told me he needed to think about it - there was some pressure not to release too much information about the outbreak.
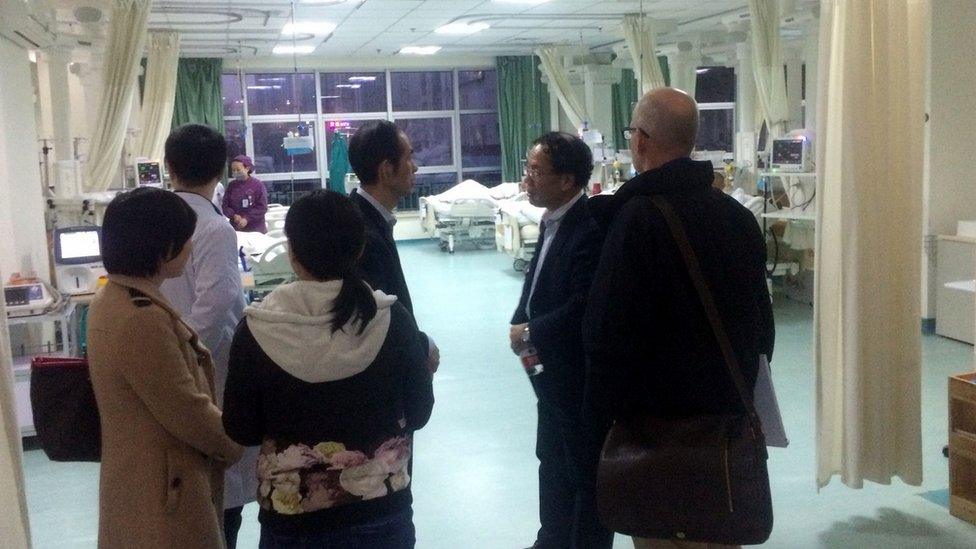
Prof Holmes (right) visited Wuhan Central Hospital with Prof Zhang and researchers from the Wuhan Centre for Disease Control in 2016
"He called me back about a minute later and said, 'OK, let's do it.'"
A researcher in Prof Zhang's lab emailed the genetic code to Eddie Holmes, who called a colleague in Edinburgh, in the UK. "It was about 01:00 there," Prof Holmes recalls.
In the ensuing 52 minutes, the scientists together wrote a brief post explaining that they were "releasing a coronavirus genome from a case of a respiratory disease from the Wuhan outbreak" and uploaded the code. Their post said that researchers should please "feel free to download, share, use and analyse this data".
With that post, the full genome of Sars CoV-2, external - the code that makes the coronavirus - was available to any scientist with an internet connection.
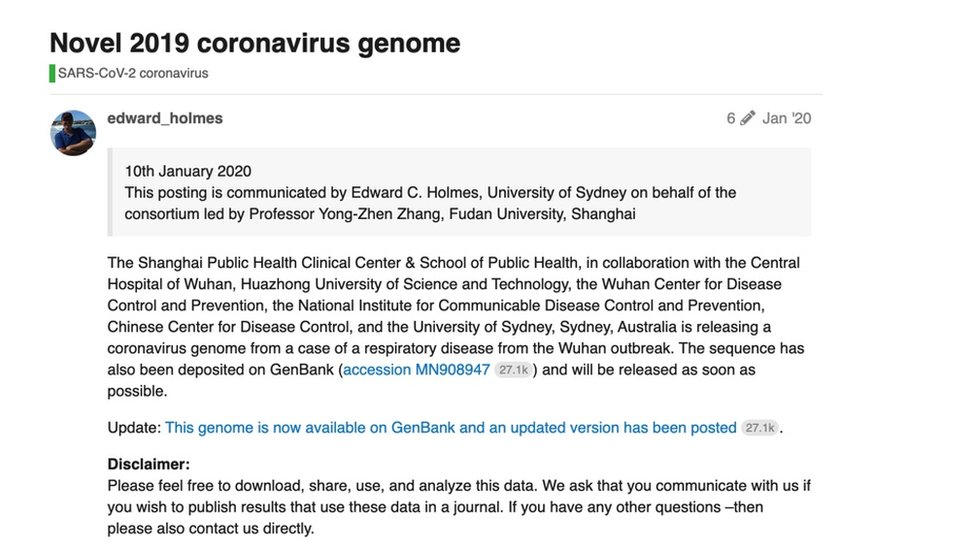
The researchers posted the full genome online on 10 January 2020
It set off 12 months of break-neck scientific endeavour. If you search the US National Library of Medicine - a database of published medical science studies - for mentions of Covid-19, external, you will retrieve more than 90,000 results.
"If we didn't collaborate with colleagues in China - if I wasn't working with and talking to Zhang - that sequence wouldn't have gone online as early as it did," says Prof Holmes.
That same weekend, scientists at a US pharmaceutical company called Moderna, which had never previously brought a product to market, downloaded the genome and started work on their experimental vaccine. Scientists at Pfizer did the same.
There are multiple methods - traditional and experimental - for making vaccines, but Moderna and Pfizer use an experimental approach based on something called mRNA (or Messenger ribonucleic acid). The terminology is a mouthful, but it describes a simpler, swifter approach to vaccine production. And it was boosted over the clinical finish line by the pandemic.

Margaret Keenan, 90, who became the first person to receive the Pfizer-BioNTech vaccine outside of a trial, in December 2020
Vaccination is fundamentally based on "showing" your immune system the disease-causing agent, so it can form a biological memory and be primed to fight it. For many existing virus vaccines, this has meant producing versions or pieces of the virus itself - often snippets of viral protein stripped of their disease-causing ability. These are grown inside chicken eggs, packaged up and injected.
"To grow enough of those things can take a very long time - sometimes years," explains Prof Robert Langer, one of the founders of Moderna, and a professor of chemical engineering at the Massachusetts Institute of Technology (MIT) in Cambridge, US. "With mRNA - you use the body as a factory to make that protein. So rather than have a giant plant with all these eggs to grow your proteins, you just make the mRNA, give it to the patient and the patient does everything else."
Messenger RNA is a short sequence of coded genetic instructions for the protein you want a cell to make.
"I've been working on this technology for decades," says Prof Langer. "This is actually the ninth mRNA vaccine that Moderna has developed." The others, which include vaccines that are designed to prime people's immune systems to fight their own cancer, are still in clinical trials.
"But when the pandemic hit, this technology lent itself to doing things as rapidly as possible."
There are now more than 150 coronavirus vaccines in some stage of development. But Pfizer's mRNA vaccine, which it developed in partnership with the German company BioNTech, was the first to receive emergency authorisation by the US Food and Drug Administration. It was quickly followed by Moderna's vaccine - less than a year after scientists first downloaded the genome it was based on.

Researcher in a laboratory at Oxford’s Jenner Institute working on the coronavirus vaccine developed by AstraZeneca and Oxford University
It was not until the end of January that the World Health Organization called the coronavirus outbreak a "public health emergency of international concern". And it took until March for the WHO to officially declare a pandemic. Efforts to understand the virus - to find out where it came from, how to treat it and learn how it was evolving - were already moving almost as rapidly as the pandemic.
"With that genome, we didn't need the actual virus," explains Dr Dalan Bailey from the UK's Pirbright Institute. "We could take that code and actually get the part of the virus that we wanted to study synthesised [using the genome as a blueprint] and have it in our lab within days.
"That definitely allowed us to start our research much more quickly."
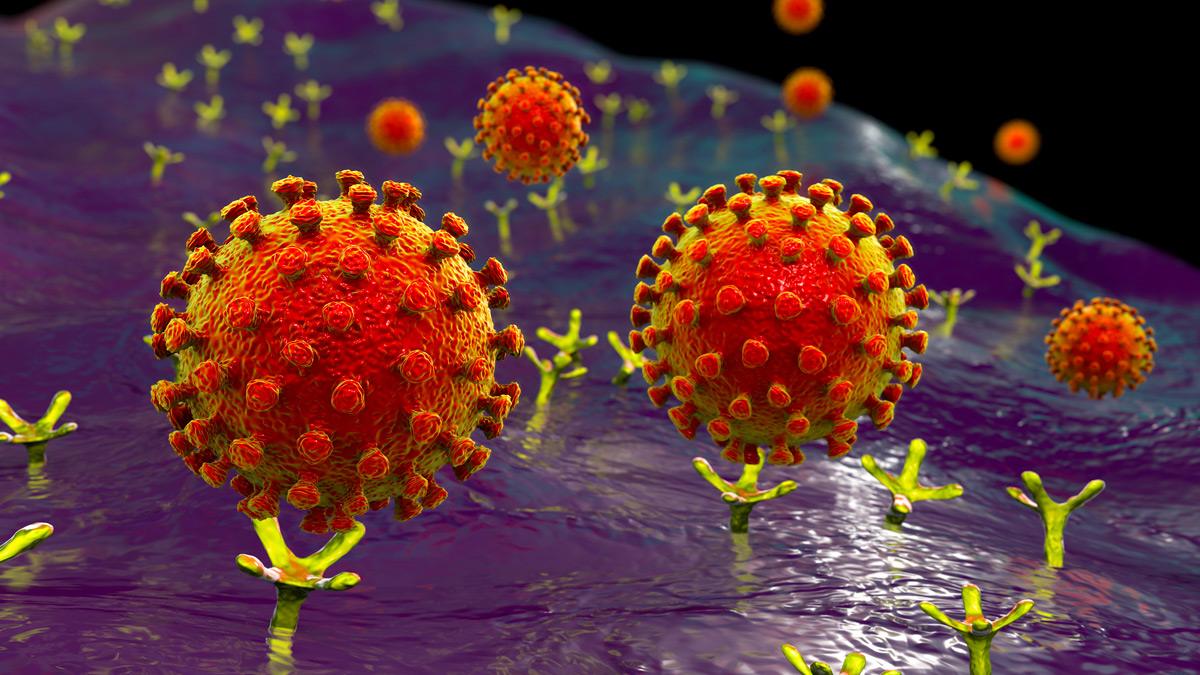
Illustration of the coronavirus binding to a human cell
Dr Bailey and his colleagues study how exactly the coronavirus latches on to and hijacks each cell. Key to this are those spikes we now see on every graphic depiction of the spherical coronavirus structure. Not only is the spike protein the key that unlocks viral entry into a cell, it is also the part of the virus that is most frequently recognised by our immune systems, so understanding its shape and its function is critical to optimising vaccines.
By the end of March, with many European countries in lockdown, Chinese scientists had mapped the exact atom-by-atom structure of that spike protein, external.
Knowing what the protein physically looks like means researchers can work out how antibodies bind to it. That is critical, because antibodies are the immune system's memory proteins. Immune cells produce antibodies to fit a specific invader - a virus that they have previously encountered in the form of a vaccine, perhaps. The antibodies then latch onto it and tag it for destruction. "So this ultimately helps us design better vaccines," explained Dr Bailey.
Throughout summer months in the northern hemisphere, lockdowns (temporarily and patchily) eased, but international tension did not. Even as US President Donald Trump sought to blame China for "unleashing a plague", scientists in the US and China carried on working together. By June, scientists from both countries had collaborated on more than 120 Covid-19 studies, external.
President Jair Bolsanaro of Brazil, which remains one of the countries hit hardest by the pandemic, made baseless, very unscientific claims that Brazilians were somehow "immune" to the disease. Meanwhile, scientists there were reported to be subsidising their own Covid research, external amid a national funding crisis.

Political tensions flared between the US and China, but scientists from both countries continued to collaborate on Covid-19 research
One large international effort, called the Covid-19 Host Genetics Initiative, external, saw investigators from research centres in Europe, Asia, and North America share data on how our own genes might affect how sick the coronavirus makes us.
There is no single, simple answer to the question of why some people have no symptoms at all, while others are made extremely ill by Covid-19. But many of these efforts to solve that puzzle have focused on the "lock" on our cells that the virus uses to gain access.
It is called the Ace-2 receptor. It is a biological lock that has evolved to be opened by chemical keys that occur naturally. That mechanism is fundamental to the biochemical machinery that is constantly whirring in healthy cells. Ace-2, for example, can be used as a cellular doorway by proteins that regulate our blood pressure.
The coronavirus, however, has cleverly (and unfortunately) evolved to fit that same lock.
Like every building block of our bodies, the exact shape and function of our Ace-2 receptors is written in our genes, giving researchers valuable clues about who is most vulnerable to Covid-19.
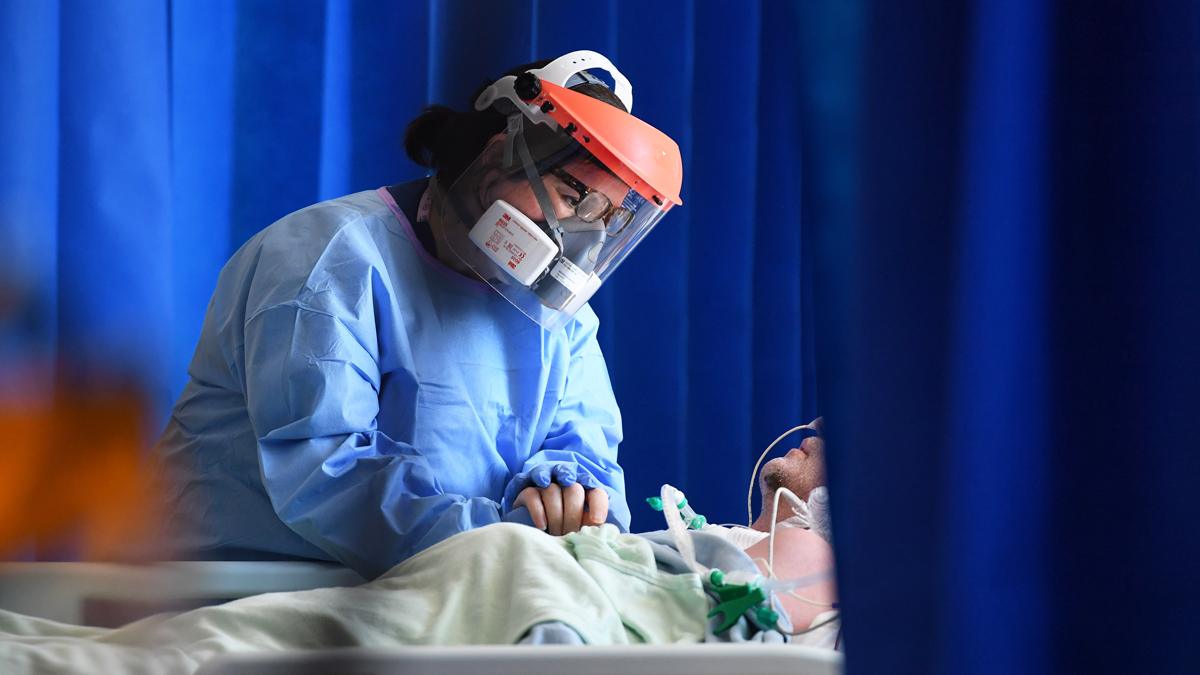
A member of staff cares for a patient in the Intensive Care Unit of Royal Papworth Hospital, Cambridge, UK, May 2020
While there is no single coded clue to predict how sick we might be made by Covid, several studies of patients have spotted differences in the genetic blueprint for an individual's Ace-2 receptor, external that might be linked with the severity of their illness.
One intriguing genetic study by German and Japanese scientists showed that a gene that we inherited from Neanderthals provides the code for an extra "biological doorway", external that the virus can use to hijack our cells.
Examining the shape of the Ace-2 receptor in other species is also providing clues about other animals that might be susceptible to the coronavirus. That knowledge will be crucial in future efforts to control the disease and prevent new strains, like the one detected in farmed mink in Denmark, from triggering further outbreaks.

Mink about to be killed on a farm at Jyllinge, Denmark, November 2020
In the midst of the pandemic, the search for any potential treatments relied, for the most part, on repurposing existing drugs. By examining treatments that were already proven safe, and by looking at the way they worked, researchers had reason to believe they had a good chance of tackling Covid. With no specific treatments available and thousands of patients critically ill, this was emergency medical research.
In May, the UK launched the world's largest clinical trial, called Recovery, external, to test a group of existing treatments.
There have been mixed results, but in June, this trial provided a breakthrough - the first evidence that dexamethasone, a cheap and widely available steroid, could reduce deaths from Covid-19. Another trial being carried out in six countries, including the UK, recently revealed another two existing drugs that could save yet more lives.

A woman wearing a mask reads a newspaper on a London Underground train, May 2020
In the long term, many scientists think we face a world where the coronavirus is always present in the global population at some level. "So we'll also need new drugs to target not only this virus, but other related viruses that could spill over in the same way," Dr Stephen Griffin, a virologist from the University of Leeds, explains.
One line of attack that looks particularly promising is a group of drugs called protease inhibitors, external.
"The main aim every virus has when it enters a cell is to hijack it and use the machinery inside to copy itself thousands and millions of times over," explains Dr Griffin. To do this, the virus tricks the cell into making its own viral building blocks, or proteins.
Some of the most important viral proteins are made as one, long chain - like a long roll of tape. So the virus also makes an enzyme called a protease that snips the sections of tape it needs to build functioning copies of itself.
"Protease is critical for the virus to thrive and drugs targeting it prevent some of the earliest stages of infection - stopping the virus from copying itself," Dr Griffin explains. Similar drugs are already successfully used against protease enzymes from HIV and hepatitis C.

Police and protesters at a mass rally against vaccination and government Covid-19 restrictions - Trafalgar Square, London, 26 September 2020
For many countries in the northern hemisphere, outbreaks resurged through autumn and into winter. Scientists have, meanwhile, tracked the virus's every move and every mutation.
By taking a swab from an infected patient, the genetic code of the exact version of the virus that has infected them can be extracted, amplified and "read" using a sequencer, which is a machine that can translate biological code into a string of letters, or nucleotides. That allows whole genomes to be compared, so specific mutations can be spotted.
"There are also huge ongoing efforts to both track mutations and to assess their impact," Dr Lucy van Dorp from University College London (UCL) explains. It was "thanks to these efforts, and to UK testing laboratories, that the recently discovered UK variant was flagged so quickly as a potential cause of concern".
Viruses mutate all the time, as they make copies of themselves in millions of infected bodies. But understanding which versions of the virus are spreading most quickly - spotting patterns in that spread - has informed public health responses and transformed contact tracing.
In countries like New Zealand, where small, specific outbreaks have been found and brought under control, contact tracers can examine and compare genomes for clues. This is how so-called superspreading events are picked up - when a cluster of infections all share the same code.
Researchers have now sequenced more than 250,000 Sars-CoV-2 genomes, which have been shared on open data platforms like Next Strain. "This is all going to feed into the vaccine design, as well," says Prof Eddie Holmes, the Sydney-based scientist who put the first viral genome online. The mRNA vaccines, in particular, are easily adaptable with a new set of genetic instructions.
Covid-19 vaccination in the refrigerator of a pharmacy laboratory in Warsaw, Poland, December 2020
Despite being able to watch it spread and evolve almost in real time, researchers still have not pinned down where the virus came from.
Scientists agree that, like ebola, Mers and Sars1, the coronavirus originated in wildlife. But most experts also agree that the animal that the virus initially "spilled over" from was not in that Wuhan seafood market.
Prof Holmes now thinks Wuhan was an "amplification" point for the virus, rather than being the origin. But there are different expert views on how the virus made the jump from bats to humans, and the lack of direct evidence currently makes solving that epidemiological puzzle almost impossible.
Chinese officials have concluded the outbreak is likely to have begun elsewhere, and that the crowded market simply helped spread the disease from person to person. "It could have come from almost anywhere in China, quite frankly," says Prof Holmes, "because Wuhan is a major travel hub."
That outstanding mystery made it all the more disappointing for infectious disease experts when China recently blocked the entry of a WHO team that was poised to spend weeks examining the evidence from those earliest cases. But connecting all the threads - from the earliest known cases of Covid all the way back to the original source of the outbreak - could take years, not weeks.
While the coronavirus genetic code still cannot tell us exactly how the pandemic started, it does show that the virus came from bats. Genetically similar viruses - probable coronavirus ancestors - have been found in colonies of bats elsewhere in China. Emerging disease researchers agree that these animals were the likely original "reservoir" for the disease. But there is a gap - possibly of decades of viral mutation and evolution - between those bat viruses and the one that is now still wreaking havoc among humans.

Closely related coronaviruses have been found in horseshoe bats
Dr Polly Hayes from the University of Westminster explains that the coronavirus could have been initially harmless when it made the first jump into humans "then... evolved to become harmful as it passed from person to person".
To answer all of those remaining questions and to have a better chance of preventing the next pandemic, Prof Holmes stresses, channels of communication between scientists in every country must remain open.
"Politics can't come into it," he adds. "Otherwise the world will be a far less safe place. I think preventing the next pandemic will be partly about fantastic, whizz-bang technology like genome sequencing and mRNA.
"But it's more importantly about people, scientists, talking to each other freely and openly."
Additional reporting by Helen Briggs. Edited by Sarah Buckley and Paul Kerley
Follow Victoria on Twitter, external
- Published21 December 2020

- Published4 May 2020