Covid vaccine: PM to have AstraZeneca jab as he urges public to do the same
- Published
- comments

The prime minister is to receive his first dose of the Oxford-AstraZeneca Covid-19 vaccine after reassuring the public it was "safe".
Boris Johnson, 56, has urged people to get inoculated and said England's roadmap out of lockdown was "on track".
He said there was "no change" to the plan despite a drop in vaccine supply.
Several European countries are to resume using the AstraZeneca jab after the European Medicines Agency (EMA) confirmed it was "safe and effective".
The regulator reviewed the vaccine amid fears about blood clots, but said it was "not associated" with a higher risk and the benefits outweighed any risks.
Mr Johnson told a Downing Street conference on Thursday that the AstraZeneca jab was safe but "the thing that isn't safe is catching Covid, which is why it is so important that we all get our jabs as soon as our turn comes".
The prime minister was himself treated in hospital for Covid-19 in April 2020 during the first wave of the pandemic.
"The way to ensure this [lockdown easing] happens is to get that jab when your turn comes, so let's get the jab done," he said.
Prof Chris Whitty, chief medical officer for England, said there were "anecdotal reports" of small numbers of people not turning up for vaccine appointments following the controversy over the AstraZeneca jab in Europe.
But he said he expected many of those would decide to get the jab after "a pause for thought", adding that Covid was still a "very dangerous disease".
"People dying, people getting significant blood clotting problems, that's one of the risks of Covid, people having long-term physical and mental effects from Covid," he said.
Boris Johnson said England's roadmap out of lockdown will not be disrupted by vaccine supply issues
Mr Johnson said England's progress towards leaving restrictions on daily life was "unchecked" by vaccine supply issues, where fewer doses have arrived from India than initially expected.
There were often delays with vaccine rollout programmes, he said, and he stressed the Indian government had not stopped any exports.
Prof Neil Ferguson, from Imperial College London, said while the delay was "disappointing" he did not think it would have "an enormous effect" on the rollout.
However, he warned the UK needed to keep variants of concern "at bay" until it could update vaccines and roll them out to the whole population.
Prof Ferguson, who is a member of the government's SPI-M modelling group, told BBC Radio 4's Today programme there should be particular concern about cases being imported from France, where infection levels are increasing.
He added that 5-10% of cases in France were the South African variant, which is of concern because it could be more resistant to current vaccines.
But Prof Ferguson suggested that adding France to the "red list" of countries, from which arrivals are required to quarantine in a hotel, would not be "practical" given the amount of essential trade between the UK and France.
Instead he said "a more bespoke arrangement" to mitigate the risks, perhaps including testing of arrivals, would be needed.
Culture Secretary Oliver Dowden said rising infection rates in Europe should act as a "wake-up call" and the travel red list was kept under "regular review".

LOOK-UP TOOL: How many cases in your area?
LOCKDOWN RULES: What are they and when will they end?
OXFORD JAB: What is the Oxford-AstraZeneca vaccine?

The European medical regulator had reviewed the AstraZeneca jab after 13 countries in Europe suspended its use over fears about blood clots in a very small number of patients.
France, Germany and Italy, along with Cyprus, Latvia and Lithuania, are to resume use of the jab on Friday while Spain, Portugal and the Netherlands will do so next week.
But Norway, Sweden and Denmark have said they will continue to pause the use of the AstraZeneca vaccine while they conduct their own independent reviews.
Like his UK counterpart, French Prime Minister Jean Castex will receive the AstraZeneca vaccine in an effort to sway a doubtful French public.
Sir Kent Woods, former chairman of the EMA, told BBC Breakfast the pause of the rollout of the AstraZeneca jab in some European countries was "unfortunate" because it could damage public confidence.
Prof Andrew Pollard, director of the Oxford vaccine group, said the EMA findings would help "rebuild confidence" and it was "reassuring" that safety monitoring was taking place.
WATCH: Pfizer v Oxford v Moderna – three Covid-19 vaccines compared
In the UK, the Medicines and Healthcare products Regulatory Agency (MHRA) also concluded that any link between the jab and clots was unproven.
The MHRA and the EMA say people can have confidence in the vaccine's benefits and should get immunised but anyone with a headache lasting more than four days after vaccination should seek medical advice, as a precaution.
The same advice applies if someone develops unusual bruising.
That is because the regulators have received a very small number of reports of an extremely rare form of blood clot occurring in the brain.
It is this type of clot that triggered some European countries to pause rollout of the Oxford-AstraZeneca vaccine.
In the UK, five cases of cerebral sinus vein thrombosis (CSVT), among 11 million people who have received the vaccine, occurred in men aged between 19 and 59. One of these was fatal.
The EMA has received an additional 13 reports of CSVT.
The World Health Organization (WHO) has called on countries to continue using the vaccine, and is due to release the results of its own review into the vaccine's safety later on Friday.
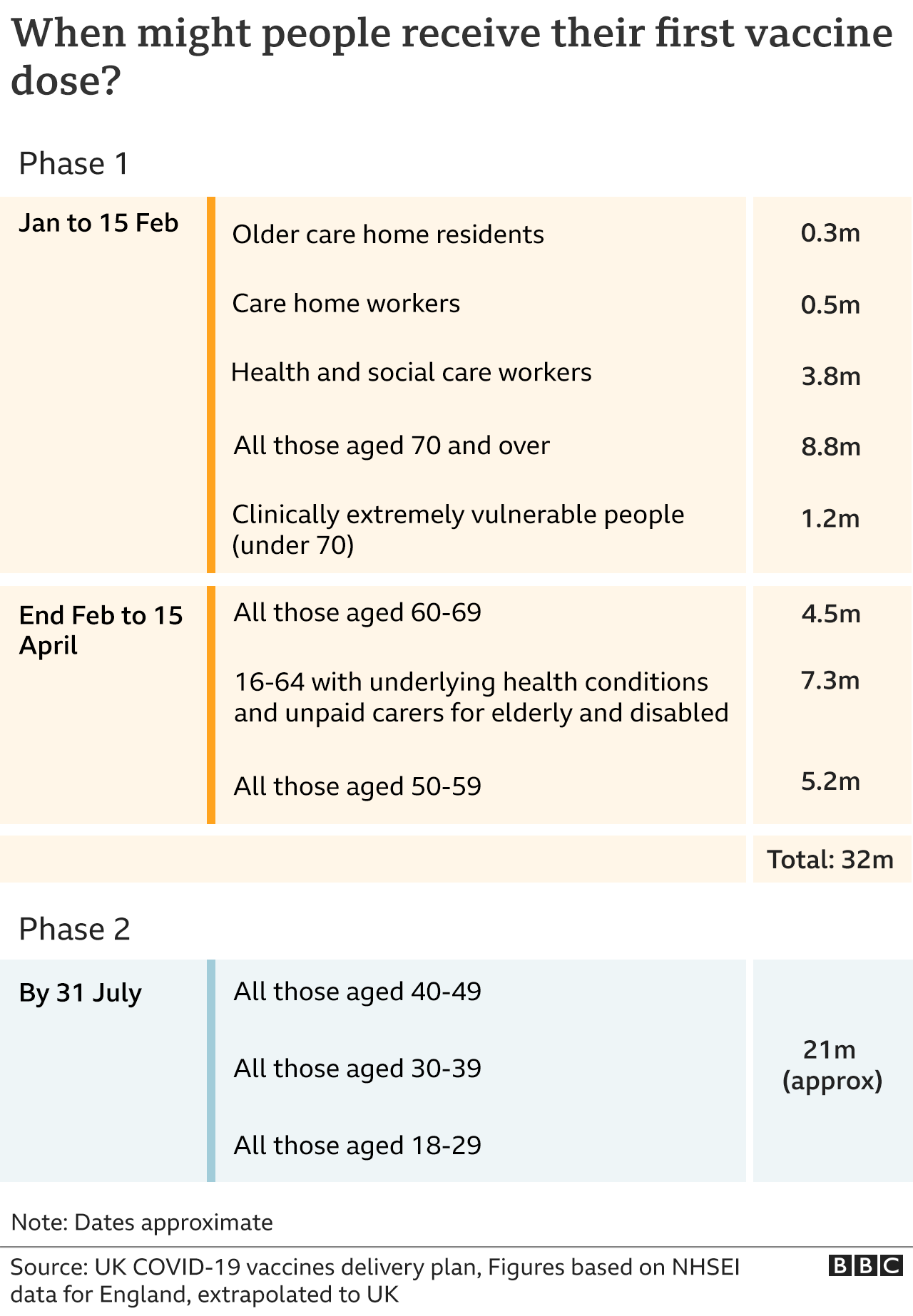
Meanwhile, the UK saw 7% more deaths than would be expected in 2020 based on the previous five years, according to new figures from the Office for National Statistics covering up to mid-December.
It experienced one of the worst death rates in Europe in the first half of 2020 but has since been overtaken by Poland, Spain, Belgium, Bulgaria, the Czech Republic and Slovenia.

LINE OF DUTY IS BACK: Get in on the action now
TEACH ME A LESSON: Why do we get ill and what does it mean for our bodies?

- Published18 March 2021
- Published18 March 2021
- Published18 March 2021