Study uncovers new applications for schizophrenia drug
- Published

The drug trifluoperazine, primarily used to treat schizophrenia, may also be able to help stroke victims, scientists in the Midlands found
A schizophrenia drug may be able to significantly reduce swelling from brain and spinal cord injuries, scientists have found.
The discovery could lead to a safe treatment for millions of people who suffer strokes, traffic accidents or sporting injuries in the future.
The team, based in Birmingham and Wolverhampton, said the results were very exciting.
Their research was published this week in the scientific journal Cell.
It is believed that because the drug trifluoperazine (TFP) has already been licensed for use in humans, it could speed up clinical trials in patients.
"Rats who had not been given the treatment were still disabled after six weeks, but those who had a single injection, can walk normally after just two weeks," lead scientist Prof Roslyn Bill, at Aston University, said.
In patients who have received a blow to the head, tiny gateways called aquaporins rush to the surface of brain cells and open up, letting in water. This makes the cells swell and puts pressure on the brain and spine.
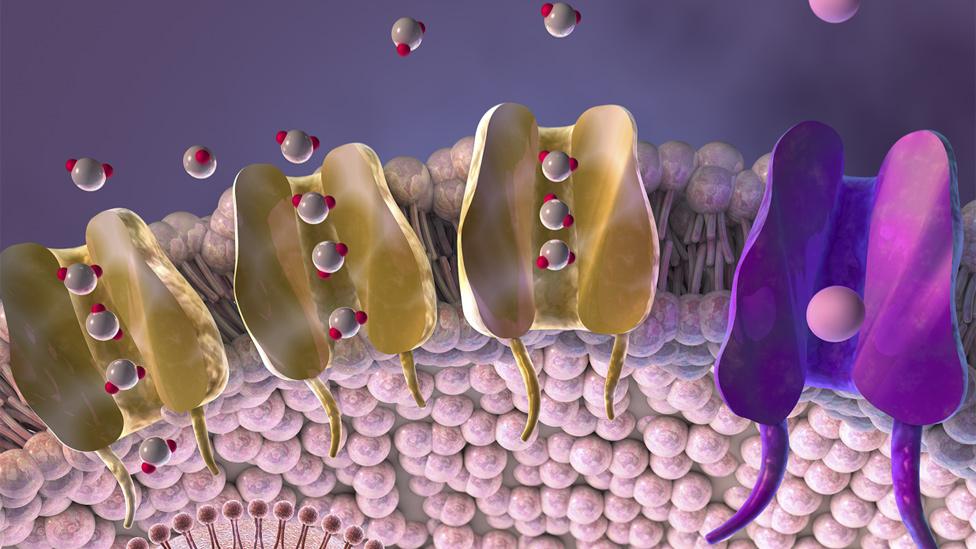
Aquaporins allow water to flow into cells, causing them to swell
The trigger for aquaporins to do this is a protein called calmodulin. The drug TFP stops calmodulin binding to the aquaporins and they do not move to the surface of the cell.
'Major advance'
Currently, patients who suffer brain injury are cooled and put into an induced coma. Sometimes, they have to be operated on and have part of their skull removed to relieve pressure on the brain.
Dr Zubair Ahmed, of the University of Birmingham's Institute of Inflammation and Ageing, said this could be a major advance, because current treatments do nothing to prevent the brain damage caused by swelling.
"The drug offers a real solution to these patients and can be fast-tracked to the clinic," he said.
The research team collaborated with scientists in the USA, Canada, Sweden and Denmark.
Prof Bill said a first-stage clinical trial would cost upwards of £100,000, but drug companies may not be interested in funding it because when a drug already exists, it's harder to make it profitable.
However, she said if successful, the treatment "could lead to significant NHS savings by reducing the need for physiotherapy and the long-term care of patients with severe brain injuries".
Scientists working at the University of Birmingham, the University of Wolverhampton and Aston University were among the global team who developed the treatment.
- Published13 January 2020

- Published9 January 2019

- Published6 April 2020
- Published20 February 2020
