Colchester baby's parents 'devastated' over lifesaving drug
- Published

Megan Willis and John Hall want their baby to have "the best chance at life"
The parents of a baby with a rare fatal genetic condition said they were "devastated" he might not be eligible for a new lifesaving drug.
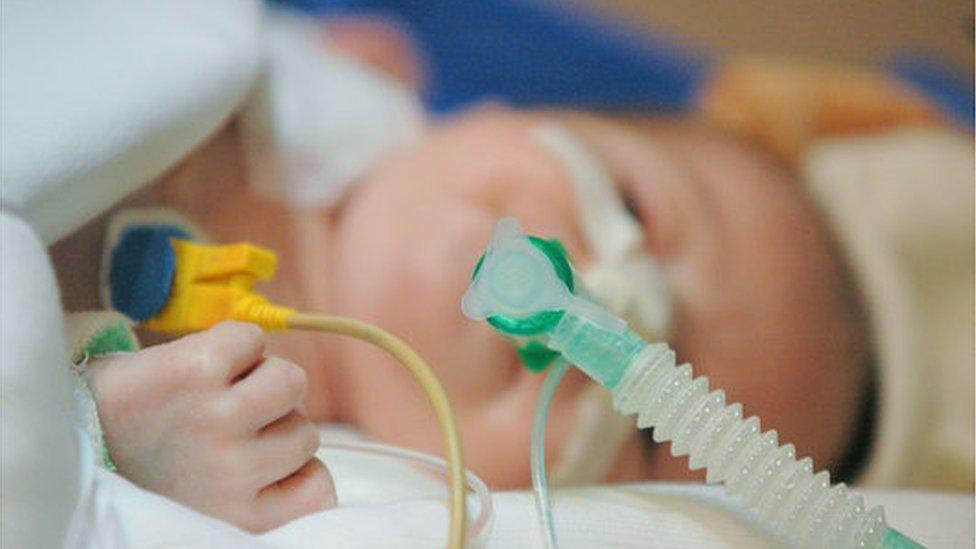
Six-month-old Edward, from Colchester, has severe spinal muscular atrophy (SMA), meaning he lacks a protein vital for muscle development.
The gene therapy Zolgensma has just been approved to treat the disorder.
NHS England said it hoped to be able to offer the treatment to patients beyond those specified in current guidelines.
Those guidelines, set by the National Institute for Health and Care Excellence (NICE), state it should only be used for babies under six months old who were not already being treated.
Edward was diagnosed at two months old and receives injections of another drug called Spinraza every four months at Addenbrooke's Hospital in Cambridge.
The drug can help slow degeneration in some SMA patients, but must be taken for life compared to the one-off injection of Zolgensma.

Megan Willis and John Hall believe it is a "race against time" for their baby
His mother Megan Willis, 29, said: "We just want the best chance at life for Edward. We are willing to fight all the way to make sure he can have this drug.
"We found out it had been approved and we were ecstatic and then we read the small print and I just burst into tears. I couldn't believe it. It seems so unfair."
About 80 babies and young children could benefit from Zolgensma each year in England, according to experts.
But a decision on whether to give it to babies older than six months and those already being treated must be dealt with on a case-by-case basis by a national team of clinicians, according to the guidance.
Ms Willis said the process of facing that panel would cause "more stress" for youngsters' families at an already difficult time.
'An absolute nightmare'
SMA causes muscle weakness and affects movement and breathing, meaning most babies who have it do not live past the age of two without intervention.
In studies, Zolgensma has helped babies breathe without a ventilator, sit up on their own and crawl and walk after a single infusion treatment.
Doctors say the drug, which contains a replica of the missing gene SMN1, needs to be given to babies as early as possible to halt the progression of the disease.
It is thought to be the most expensive drug in the world, at a cost of £1.79m per patient, though NHS England said it had negotiated an undisclosed discount.
Edward's family had been fundraising to send him to the US to have the treatment before it was approved in England.
Ms Willis said it would be cheaper in the long run to treat Edward with it, because it was a one-off transfusion rather than multiple injections a year for the rest of his life.
"This is a race against time for us. He needs to have this treatment as soon as possible. Our doctor has been fantastic and said she will try her best to make sure he is eligible, but she can't guarantee anything," she said.
"These past few months have been an absolute nightmare for us and just when we think we have got some light at the end of the tunnel, it has been snatched away," she said.
A spokesperson for NHS England said: "The landmark deal to provide Zolgensma to NHS patients goes further than NICE recommendations, meaning that children with type 1 SMA older than six months and who may have been previously received other treatments will be considered for treatment within the terms of the drug's European licence.
"SMA is a complicated and rare disease and the decision will be taken by clinicians, as part of a specialist multi-disciplinary team."

Find BBC News: East of England on Facebook, external, Instagram, external and Twitter, external. If you have a story suggestion email eastofenglandnews@bbc.co.uk, external
- Published8 March 2021
- Published17 December 2020

- Published15 May 2019
