Cystic fibrosis parents in Orkambi drug price plea
- Published
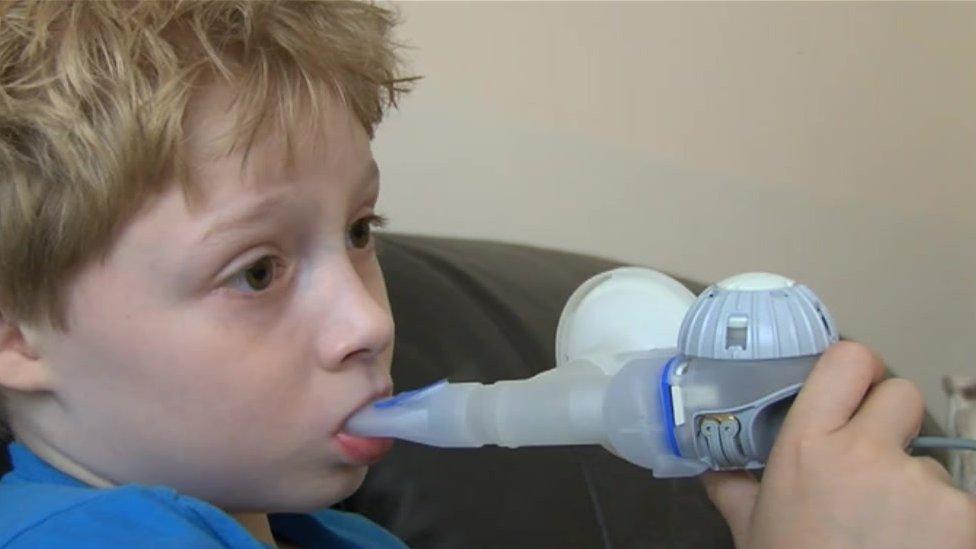
Luis Walker wrote to the drug company in August
A mother has come face-to-face with the pharmaceutical company she says is pricing her son out of the drug that would be his "life-saver".
Eight-year-old Luis Walker has cystic fibrosis and last month told Vertex Pharmaceuticals that Orkambi would make him "feel much better".
Vertex currently charge £100,000 for a year's treatment with Orkambi.
Christina Walker joined other parents in London to urge the company to reduce the price ahead of talks with the NHS.
The National Institute for Health and Care Excellence (Nice) has said the price offered by Vertex was unsustainable.
In a handwritten letter to Rebecca Hunt, Vertex vice-president for corporate affairs, in August, Luis asked for the price of the drug to be lower so that it could be made available on the NHS.
In response, the company said it was hoping to meet the NHS again, with the key talks now scheduled for Thursday.

Christina Walker met representatives of Vertex with other parents
Mrs Walker travelled to London from the family home in Horam, East Sussex, with other parents of children with cystic fibrosis on Wednesday for a question and answer session with Vertex.
"At this point any further delays would be unforgiveable and we really urge both parties to see what's important in this, and that's patients getting the drugs to make them well," she said.
Earlier this year, Vertex said it had made the "best offer in the world" to NHS England.
Orkambi was approved by the European Medicines Agency in November 2015.
Vertex rejected an NHS five-year deal which provided the potential for Vertex to secure revenues from the NHS in the region of £500m over the next five years and in excess of £1bn over the next 10 years.
Mrs Walker said the UK was now "falling behind 13 other countries, mostly in the EU" in providing Orkambi.
Orkambi has been shown in clinical trials to improve lung function and respiratory symptoms in people with the life-shortening condition, and is thought to be appropriate for about 50% of sufferers.
- Published15 August 2018

- Published20 July 2018

- Published16 May 2018

- Published21 April 2018

- Published20 March 2018

- Published19 March 2018

- Published6 September 2017

- Published30 November 2016
- Published24 March 2016
