Thousands campaign for cystic fibrosis drug Orkambi to be made available on NHS
- Published
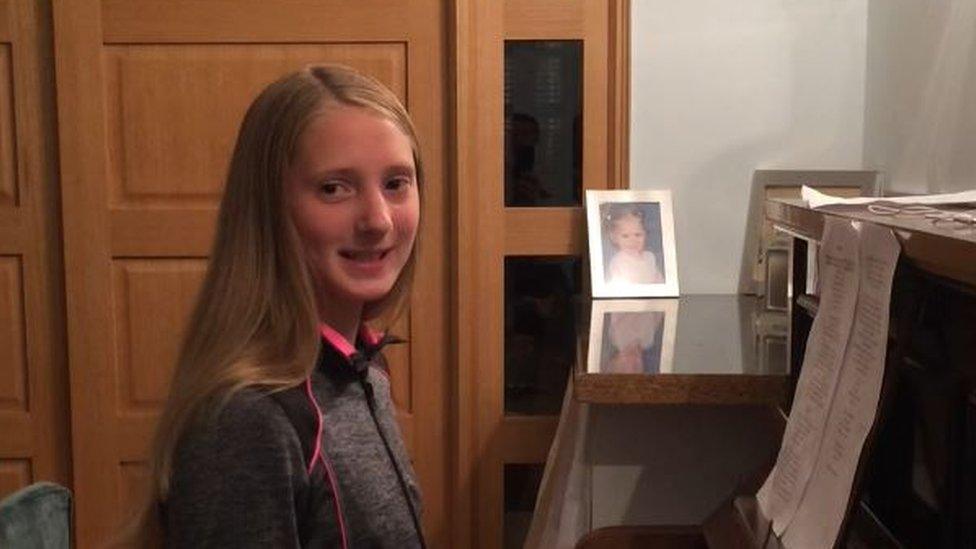
Holly Cramner has to take up to 30 pills a day to strengthen her lungs due to cystic fibrosis
A father is among thousands of people campaigning to make a new cystic fibrosis drug available on the NHS.
Kevin Cramner, from Inglewood, Berkshire, said access to Orkambi would "massively reduce" his 12-year-old daughter's dependence on antibiotics.
The National Institute for Health and Care Excellence (NICE) said the drug was not "cost effective" as there was no long-term data available.
It costs more than £100,000 a year for each patient.
Cystic fibrosis is a life shortening genetic condition.
Mr Cramner said his daughter Holly has to take up to 30 tablets daily and keep up a regime of physiotherapy and athletics to stave off the effects, but her lung function is still below that of a healthy child her age.
The Cystic Fibrosis Trust said new data released since NICE's judgment shows Orkambi almost halves the rate of decline in lung function in people with cystic fibrosis over a two-year period, and could benefit over 3,000 patients.
More than 6,000 people have written to their MPs over the issue.
'Death sentence'
Mr Cramner said: "There are children worse off than Holly and actually it's a death sentence for them not having this drug."
James Barrow from the Cystic Fibrosis Trust said: "Half of people with cystic fibrosis don't live to see their 28th birthday and this drug has been seen to slow down the progression of the illness by almost half."
He said NICE should fund treatment for patients in Britain so it could monitor the long-term effectiveness of the drug before negotiating payment with the drug's manufacturer, Vertex.
The Cystic Fibrosis Trust is working with MPs to hold a Parliamentary debate on 13 December on the issue.
Prof Carole Longson, director of the NICE Centre for Health Technology Evaluation, said: "For the benefits it offers, the cost of Orkambi is too high."
- Published24 March 2016

- Published17 May 2015
