Prostate cancer drug decision 'grossly unfair'
- Published
Prostate cancer is the most common cancer for men in Scotland
A charity has said it is "grossly unfair" that men with advanced prostate cancer in Scotland will not be able to get a treatment on the NHS that could extend their lives.
The Scottish Medicines Consortium (SMC) has rejected cabazitaxel chemotherapy for routine use by the NHS.
It has been approved for use in England and Wales by the National Institute for Health and Care Excellence (NICE).
Prostate Cancer UK said the decision was "baffling".
SMC chairman Prof Jonathan Fox said: "Unfortunately, the committee was not able to recommend cabazitaxel for prostate cancer as the overall health benefits offered by the medicine did not justify its cost.
"Discussions around next steps have been held between SMC and the company, and we would welcome a resubmission reflecting the points raised in our assessment."
'Extra time'
Prostate cancer can affect one in 11 men in Scotland, making it the most common cancer for males.
Nearly 11,000 men across the UK die from the disease each year.
Heather Blake, of Prostate Cancer UK, said that for some people who have prostate cancer cabazitaxel chemotherapy was "the only remaining treatment choice after other treatments have stopped working".
She added: "Today's decision robs these men in Scotland of precious extra time with loved ones while men in a similar situation in England and Wales are given access.
"This is grossly unfair and baffling given that the information presented at both appraisals was the same.
"We therefore urge the SMC and the manufacturer to do everything in their power to get this treatment approved."
Rob Jones, professor of clinical cancer research at the Institute of Cancer Sciences, University of Glasgow, said: "Despite recent advances in the treatment of advanced prostate cancer, clinical trials conducted in Glasgow and elsewhere have demonstrated that cabazitaxel is a life-prolonging treatment for some patients."


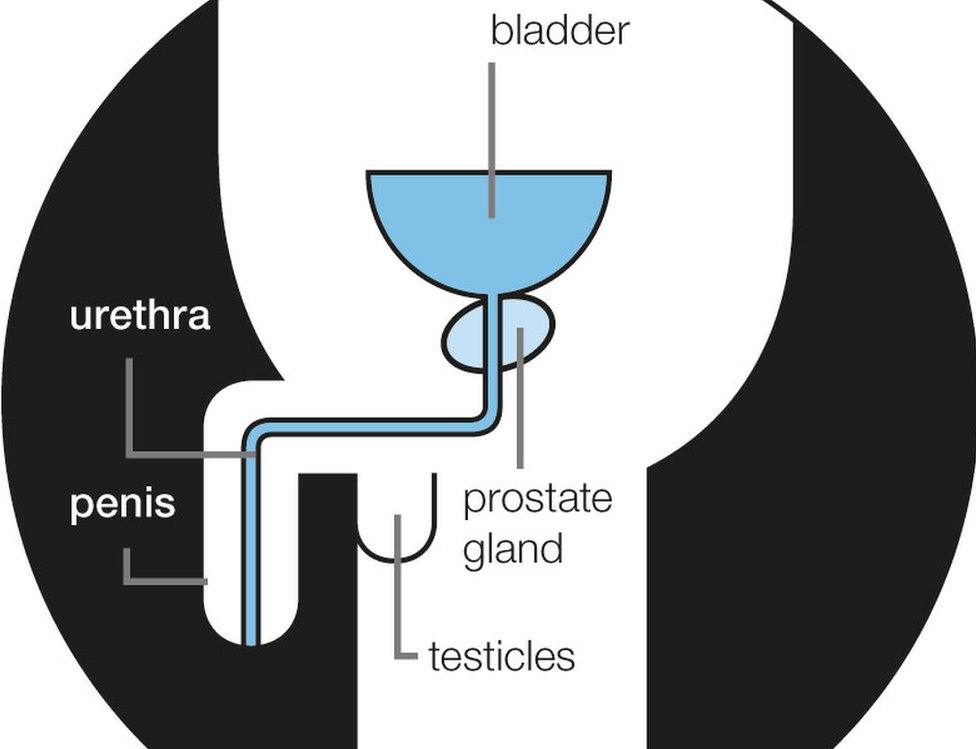
Prostate cancer symptoms
needing to urinate more often, especially at night
needing to run to the toilet
difficulty in starting to urinate
weak urine flow or taking a long time while urinating
feeling your bladder has not emptied fully

While the SMC rejected cabazitaxel, it approved new treatments for advanced Parkinson's disease, acute lymphoblastic leukaemia and severe asthma, among others.
Katherine Crawford, of the charity Parkinson's UK, said she was "delighted" the SMC had given the go-ahead for co-careldopa - which is also known as Duodopa - to be used by the NHS.
She added: "Parkinson's specialists will now be able to prescribe Duodopa without having to apply to their NHS board each time. This means fewer delays and less potential for a postcode lottery.
"Duodopa can enable people with advanced Parkinson's to live independently, to socialise and take part in activities they enjoy again."
- Published22 April 2016

- Published2 May 2016
- Published21 March 2016

- Published22 May 2015

- Published1 November 2013