Wales NHS to offer MS cannabis drug Sativex
- Published
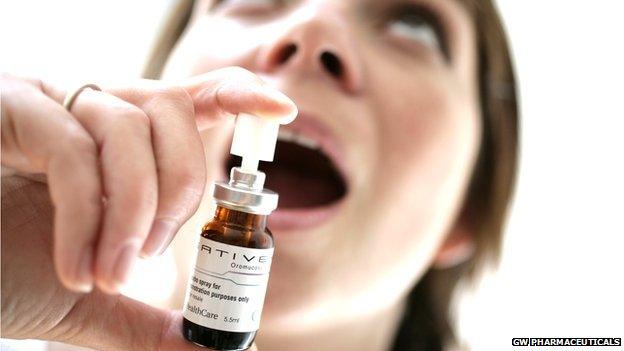
Sativex is the first cannabis-based medicine to be licensed in the UK
The NHS in Wales will be the first in the UK to fund a cannabis-based medicine for people with multiple sclerosis.
Sativex is taken as an oral spray and has been approved by the All Wales Medicines Strategy Group (AWMSG).
It will be available on prescription to treat muscle spasms for MS patients who have not responded to other medicine.
The MS Society said Wales was leading the way in the treatment.
Its programme director for policy, Sally Hughes, added: "Muscle spasms and stiffness in MS can be painful and distressing and so the availability of a treatment that can potentially alleviate these symptoms is good news.
"We particularly welcome this decision considering the draft NICE (National Institute for Health and Care Excellence) clinical guideline, published in April, rejected this treatment for use on the NHS in Wales and England based on a flawed assessment of the drug's cost effectiveness.
'Ease suffering'
"For some time we've been aware of people in Wales paying privately for this licensed treatment; this decision should make life a lot easier for them."
Sativex is the first cannabis-based medicine to be licensed in the UK.

Tony Wiggins, chairman of the Cardiff and Vale MS Society, has trialled Sativex and called it a "tremendous step forward".
"It's good for spasms and other effects of MS - and it does work," he said.
"And if a treatment works then it should be made available."
Wales Health Minister Mark Drakeford said: "Following the appraisal of Sativex by the All Wales Medicines Strategy Group, I am pleased to announce we will be making the medicine available on the Welsh NHS to those who need it.
"I hope this decision will help ease the suffering of some of those who have to live with the reality of MS everyday."
Director of service development at the Multiple Sclerosis Trust, Amy Bowen, said: "We are extremely pleased that people with MS in Wales will finally have better access to Sativex.
"As a charity we have campaigned over a long period for Sativex to be widely available because of the significant impact that MS spasticity can have on daily activities.
"We just hope that this recommendation will now lead to Sativex being more easily accessible in the rest of the UK."
- Published22 July 2014

- Published2 September 2011
- Published23 May 2011

- Published14 September 2010