Covid: Oxford-AstraZeneca vaccine rollout to start in Wales next week
- Published
Brian Begg was one of the volunteers who took part in the trials earlier this year
The rollout of a second Covid-19 vaccine across Wales will begin next week, the Welsh Government has said.
The coronavirus vaccine designed by scientists at the University of Oxford was approved for use in the UK on Wednesday.
The UK has ordered 100 million doses from the manufacturer AstraZeneca - enough to vaccinate 50 million people.
Health Minister Vaughan Gething said it was not "a quick fix" and the impact will not be seen for months.
Mr Gething said he would be "delighted" if there was population coverage by Easter, but said he did not want to "give out false hope", adding: "By April, if the weather is improving, it will allow us to do more things outside."
Meanwhile First Minister Mark Drakeford urged people to keep following rules, adding: "If people think that now we have a vaccine, everything will be ok... it's going to turn good news into bad news."
Two doses will be needed, with an interval of between four and 12 weeks between doses.
BBC Wales understands that about 20,000 doses are expected to be available in Wales from next week, with the hope this will increase to about 100,000 by the end of January.
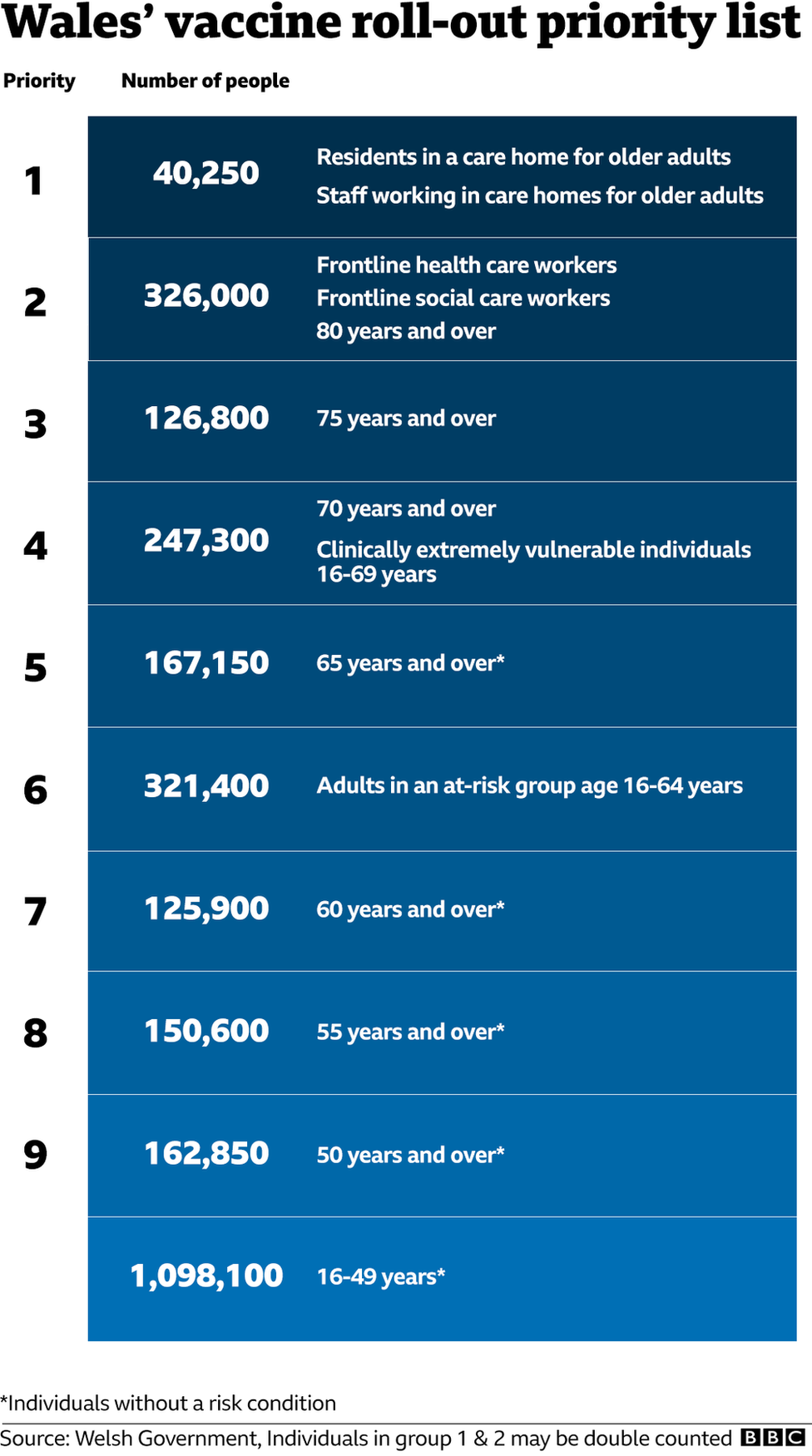
Those at highest risk of severe symptoms from Covid-19 - such as the elderly or those with certain medical conditions - will be first to get the new vaccine, before it is rolled-out to other groups.
The vaccine has been deemed to be safe and effective following large-scale clinical trials
Wales is set to receive its population share of the 100 million doses over the next weeks and months.
Mr Gething said the fact it can be stored in a fridge meant it could be rolled out in care homes and from GP pharmacies far more easily than the Pfizer vaccine which has to be kept below -60C.
There has been criticism about the rollout - results up to 23 December put Wales behind the other UK nations with 0.72% of the population vaccinated and 22,595 doses administered.
"We understand there are high expectations and excitement at the arrival of a second vaccine," Mr Gething added.
"However, it will take time to reach everyone as this is not an instant fix. We won't receive all the doses at once and we have to be realistic about the scale and pace of delivery when we are vaccinating the entire adult population.
"We will not see the impact of the vaccine for some months and the pressure on the NHS will continue during this winter. It is essential that we all continue to play our part and do the right thing to protect each other."
He added "nobody will be left behind" by the Welsh NHS, and people were asked to wait to be invited for the vaccination.

Dr Robin Howe, incident director at Public Health Wales, said: "Although we are right to celebrate this announcement, we would sound a note of caution because vaccinating the adult population of Wales is a significant task, and the vaccine will take time to reach everyone.
"Please do not to phone your GP, pharmacy or hospital asking when you will get a vaccine. When someone is in one of the groups eligible for the vaccine, they will be invited to attend a dedicated clinic which will have been set up to ensure patient safety and that of the healthcare professionals."
He added: "The effects of the vaccines may not be seen nationally for some time, and with Wales at alert level four we must continue to follow the advice on keeping Wales safe."
'Race against time'
The vaccine was described as "a game changer" by Mario Kreft, the chairman of Care Forum Wales, which represents care and nursing homes across Wales.
"The concerning issue now is the new mutant strain of this virus that seems to be spreading right across our communities and appears to be out of control so all care providers are trying to ensure now that they are ready for this vaccine," he said.
"It can't come soon enough - it's a race against time."
Aneurin Bevan University Health Board recruited almost 450 participants in the vaccine's trial, after calling for volunteers aged over 18.
Assistant director of research and development Jeanette Wells said: "We are particularly proud to have been able to contribute to the national effort in this way, and very much look forward to seeing this vaccine deployed."
At the beginning of December, the Pfizer BioNTech vaccine was the first to receive MHRA approval in the UK, with 40 million doses made available for delivery across the UK.
Rollout has begun to front-line health and social care staff, as well as care home residents and staff and people aged over 80.
In the first two weeks, 22,000 people were vaccinated, and the Welsh Government believe about 30,000 have now received it.

Vaccinations started earlier in December after regulators approved the Pfizer/BioNTech vaccine
Unlike the Pfizer BioNTech vaccine, the Oxford AstraZeneca vaccine is stored at normal vaccine fridge temperatures.
This means it will have few storage and transportation issues, making it much easier to use in community settings such as care homes and GP surgeries.
Dental surgeries, opticians and pharmacies may also be used to administer the vaccine, to help speed up the process.
Experts now hope to administer as many initial doses of the immunisation to the population as possible, meaning NHS and care workers, who had already received their first vaccine, may have to wait longer for their second dose.
Welsh Secretary Simon Hart said it "takes us a step closer to our normal lives".
The Conservative MP added: "Like the Pfizer/BioNTech vaccine, the UK government has procured and paid for millions of doses of the Oxford/AstraZeneca vaccine for all parts of the UK, and with vaccine production taking place in north Wales it will put Wales on the frontline of the global stage in the fight again the Covid pandemic."
Plaid Cymru's health spokesman Rhun ap Iorwerth called for assurances over its effective rollout.
"Wales has been behind every other UK nation in terms of numbers vaccinated, and this needs to be addressed with urgency," he said.
Vaccine produced in Wales
A laboratory in Wrexham will produce the vaccine, with Prime Minister Boris Johnson saying it could provide "salvation for humanity" following a visit.
Wockhardt UK entered an agreement in August to help prepare the vaccine for distribution.
Managing director Ravi Limaye said: "We are incredibly proud to be fill finishing the approved AstraZeneca Covid-19 vaccine and play our part in the fight against this global pandemic, not to mention the positive impact it will have on the Wrexham business community."
Senior responsible officer for Wales' vaccine programme Dr Gillian Richardson said it was "great news" a second vaccine had met "strict standards of safety, quality and effectiveness".
Dr Nicola Williams, of Health and Care Research Wales, which is nationally co-ordinating research and studies in Wales, said: "Our research community is working hard to provide the evidence we need to fight this pandemic and the approval of the Oxford AstraZeneca vaccine is an important step forward.
"We have two further vaccines being tested in Wales right now, with more trials due to be set up in the coming weeks and months."
- Published23 November 2020

- Published22 December 2020

- Published23 December 2020