Covid: Delayed Pfizer vaccine gap 'unacceptable' says BMA Cymru
- Published
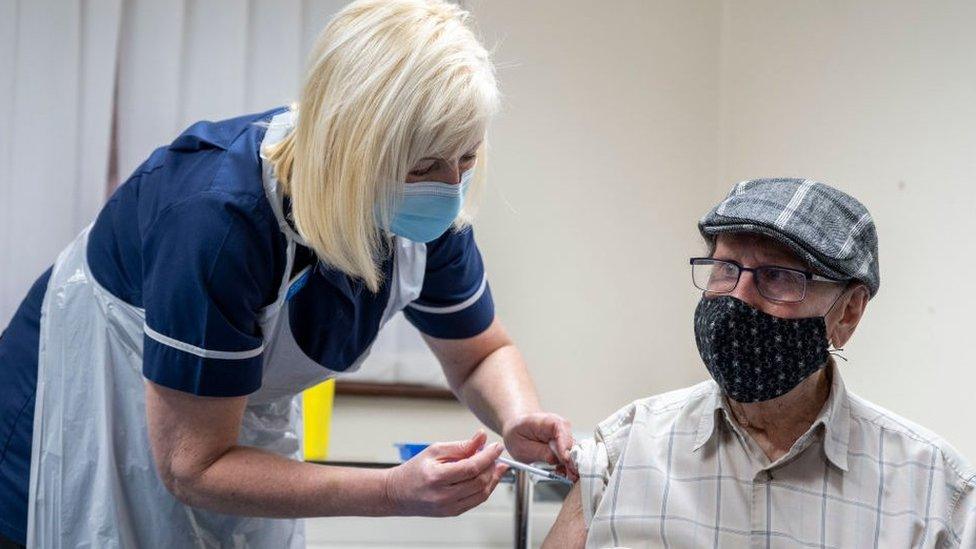
Gwerfyl Gregory receives her first dose of Pfizer vaccine at Ty Doctor in Nefyn
Senior doctors in Wales warn a delayed gap between the first and second dose of the Pfizer-BioNTech vaccine would be "entirely unacceptable and potentially dangerous".
The British Medical Association Wales (BMA Cymru) has joined calls for the 12-week wait to be halved.
It comes as a pilot started in three communities, aiming to vaccinate thousands of people this weekend.
The Welsh Government said "significant progress" had been made.
Dr David Bailey, chairman of the BMA's Welsh Council, said doctors in Wales "remain extremely concerned" by the Welsh Government's decision for a maximum of 12 weeks between the first and second doses of the Pfizer vaccine.
He said it was known the second dose provided an "excellent amount of protection" when given after three weeks but there was no evidence about how effective a second dose will be after 12 weeks.
He added: "In fact, there is worrying news from Israel which suggests that a delayed second dose may give less protection.
"This is entirely unacceptable and potentially dangerous for both staff and patients."
BMA Cymru called for all front-line healthcare staff to be given "the maximum protection possible so that they are safe to treat patients in the NHS".
"It should be clear to Welsh Government that this means giving both doses of the vaccine within the time period specified by the manufacturers," said Dr Bailey.
He added that no other European country was following the same strategy as the UK and that the BMA's position was supported by the World Health Organization and the European Medicines Agency.
"Welsh Government, along with Scotland and England, are clearly swimming against the tide and must urgently return to the approved timeframe for the second Pfizer dose so that we can reduce the stress on the NHS and fight the pandemic," he said.

Dr Phil White of BMA Cymru called for the gap to be halved between the first and second dose
Dr Phil White, chairman of BMA Cymru's Welsh GPs committee, told BBC Wales: "There's been considerable disquiet among BMA members across the country regarding this decision to extend the gap to 12 weeks.
"We're looking for a half-way house, say 42 days, which is six weeks, which is halfway to what the government propose."
Evidence query
He added there was concern over recent research that "does suggest the first dose is not as effective as was originally thought and may also not prevent you from spreading the disease, though it may prevent you from having major symptoms".
Plaid Cymru MS for the Rhonnda, Leanne Wood tweeted her support for shortening the gap.
She added she had written to Health Minister Vaughan Gething expressing constituents' concerns and asking for the evidence on which the decision was made to extend the wait between the two doses.
Allow X content?
This article contains content provided by X. We ask for your permission before anything is loaded, as they may be using cookies and other technologies. You may want to read X’s cookie policy, external and privacy policy, external before accepting. To view this content choose ‘accept and continue’.

The Pfizer-BioNTech vaccine needs to be stored at a very low temperatures and then prepared and given to a patient within 15 minutes.
It is considered less practical to give in GP surgeries and has so far been given in larger centres.
A pilot programme for rural GPs is set to vaccinate about 3,000 patients in Nefyn in Gwynedd, Buckley in Flintshire and Bridgend this weekend.
Under the trial the new community vaccination centres will open to give over-80s and those with mobility issues the jab to avoid people having to travel miles to get the jab or wait weeks to have their first dose.

Patients queue outside the Ty Doctor surgery in Nefyn
Dr Eilir Hughes, GP cluster lead for Dwyfor, who helped organise the pilot in his area, said it was already going "really well".
"We've hit our target of vaccinating hundreds of people this morning. We'll do the same this afternoon and again tomorrow. The total will be around 1,200 people," he said.
"The patients are coming from three practices, but it's better to do it from one site logistically."
'Benefit within a week'
Asked about the difference of opinion between the BMA and health officials over the period between the first and second dose of the Pfizer vaccine, Dr Hughes said: "My opinion is if the vaccine is there, don't let it rest in stockpiles.
"We need to get into people's arms because the first dose does provide you with some benefit within a week, that's what the studies from Pfizer have shown."
GPs in England started giving the Pfizer-BioNTech vaccine to patients in December. Dr Hughes said he had been in discussions with colleagues in England.
"I understand we need to do it as safely as possible and we've worked tirelessly this week to ensure we're doing everything in our means to make sure it's safe, but also efficient," he said.
"And that's where I think we're winning - it's an efficient system and convenient for our patients."
"I think it's a proven concept. We've succeeded in doing it here this weekend and I think there will be no reason why you can't replicate what we've done here across Wales.
"As long as you have the means and the will of the community teams I can't see why it can't be replicated in other areas."
Where are Wales' vaccination centres?
A Welsh Government spokesperson said: "We have made significant progress in building vaccination infrastructure across Wales to deploy both the Pfizer and AstraZeneca vaccines."
They added that seven vaccination centres - one in each health board area - were initially set up and that number grew to 14 and is currently at 28.
"We're now increasing the number of vaccination sites closer to people's homes," they added.
"We already have over 100 GP practices offering the vaccination with the expectation that we will exceed the 250 practices committed to in our strategy by the end of January.
"Our approach has been to ensure our vaccination locations to maximise the speed of roll-out, ensure safety, meet the needs of the characteristics of the vaccines and be as conveniently located as possible.
"We also want people to feel comfortable in being vaccinated, and to do this as conveniently as possible, especially for those most at risk from the harms of coronavirus."
- Published23 January 2021
- Published23 January 2021