Covid: Welsh volunteers join booster vaccines trial in Wrexham
- Published
- comments

Volunteers have been getting booster vaccines as part of the trial in Wrexham
Volunteers in Wrexham have been given a third Covid vaccine as part of a UK-wide trial.
They attended a special clinic in the town to receive their booster dose.
Wrexham is one of 18 sites across the UK to take part in the Cov-Boost study, with more than 2,800 volunteers required.
The trial will provide data on patients' immune response to a third dose ahead of a potential rollout later this year.
It is the pandemic's latest clinical study in Wales, where more than 45,000 people have volunteered to take part in various trials since March last year.
The trial will also assess acquired immunity against coronavirus variants, including the more transmissible Delta variant first identified in India.

Orod Osanlou is particularly interested in volunteers over 70 and any who has had the Oxford-AstraZeneca jab
Orod Osanlou, principal investigator for the study, said: "We now have vaccines that are safe and effective in preventing Covid-19."
"What we are now looking for with Cov-Boost is for a booster vaccine, and how this booster affects people's immune response and their protection against the virus. And, excitingly, Cov-Boost is the first study in the world that is looking at this."
The study will assess the immune responses offered by seven different types of vaccine.
Volunteers are being recruited within a 50-mile (80km) radius of Wrexham Maelor Hospital.
They are expected to attend the clinic five times over the course of a year, and will be compensated for their time.
Dr Osanlou said: "We need people who have had two doses of the vaccine and are aged over 30, and who had been vaccinated by the end of March, to take part.
"We are particularly interested in anybody aged over 70 and anybody who has had the AstraZeneca vaccines."
Sue Cusack from Montgomery in Powys was one of the first to receive a booster jab in Wrexham this week.
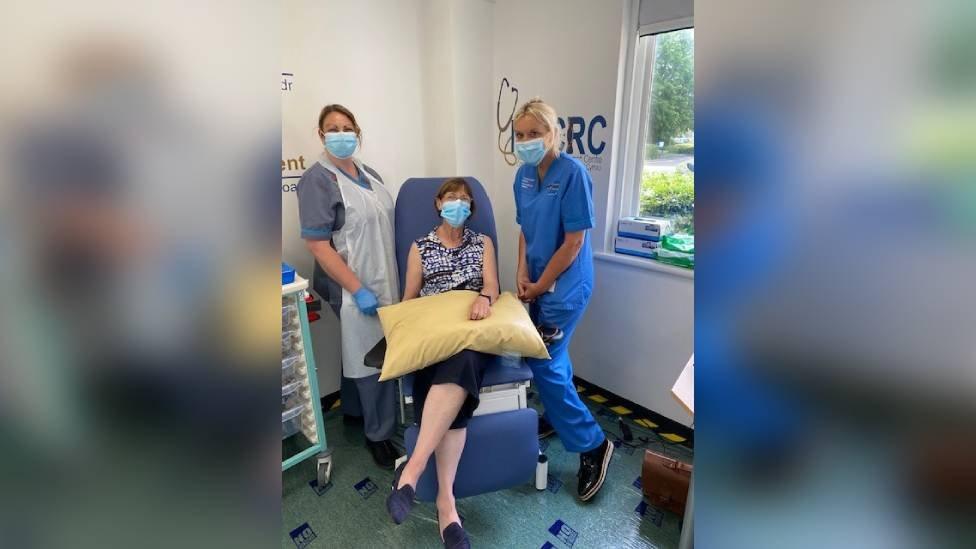
Sue Cusack was one of the first to receive a booster jab in Wrexham this week
"I wasn't nervous, it was more exciting really," she said. "It felt special to be part of the process which means that other people can have it in the future."
She said: "There is just the one place in Wales that is offering it. It was quite accessible to get there, and it's nice that Wales is taking part as part of the UK, and as part of the bigger process."
Nicola Williams, director of research, support and delivery for Health and Care Research Wales, which has co-ordinated the trials, thanked those who have volunteered.
"It has been an amazing response," she said. "They have been really critical in understanding what treatments work, what treatments don't work, and getting the right sources of information so that we can help support the pandemic response."
The outcome of the Cov-Boost trial is expected to be known by September, and will inform the further rollout of the UK's immunisation programme.
How does the trial work?
Volunteers will be randomly given one of the trial vaccines or the meningitis vaccine, which is the control.
One booster will be provided to each volunteer and it could be a different brand to the one they were originally vaccinated with.
Vaccines being trialled include Oxford-AstraZeneca, Pfizer-BioNTech, Moderna, Novavax, Valneva, Janssen and Curevac.
All volunteers will be monitored for adverse events and will have blood samples taken to check their immune response.
- Published5 May 2021

- Published8 February 2021
- Published19 May 2021

- Published8 February 2021
- Published4 July 2022

- Published7 June 2021

- Published2 April