Spinal Muscular Atrophy: Indians who need a $2.1m drug to fight rare disease
- Published

Erhan was diagnosed with SMA type-1 when he was nine months old
The Gufrans are paying a high price to keep their children alive.
Affan, seven, and Erhan, five, have Spinal Muscular Atrophy (SMA) - a rare genetic disorder that causes muscle deterioration and affects breathing. Physiotherapy appointments alone cost the family 40,000 rupees ($480; £395) a month, and their sons need constant care as they cannot sit, stand or walk independently.
"We want to try gene therapy for our sons, but one dose alone costs around 175m rupees ($2.1m; £1.7m). We simply can't afford it," says Zeba Gufran, their mother.
Zolgensma gene therapy, which the Gufrans want to try, is one of the most expensive drugs in the world. It is given as a one-time dose, usually to children under two - but the Gufrans are desperate and hope for a miracle.
Like them, many parents in India cannot afford to buy Zolgensma and other SMA drugs. While there is no official data on the number of Indians with the disease, existing literature shows that SMA affects nearly 1 in 10,000 live-born babies, external - according to one study, one in 38 Indians are carriers of the faulty gene that causes SMA, compared with 1 in 50 people in the West.
While treatments for rare diseases are expensive everywhere, the government or health insurers cover the cost in some countries. The UK has made SMA medicines available through the National Health Service; Australia offers eligible patients subsidised access to expensive life-saving drugs.
In India, patients often turn to crowdfunding to access these treatments. But as the spotlight grows on rare genetic diseases, patients and their family members are coming together to put pressure on the federal government to bring down the cost of these drugs.
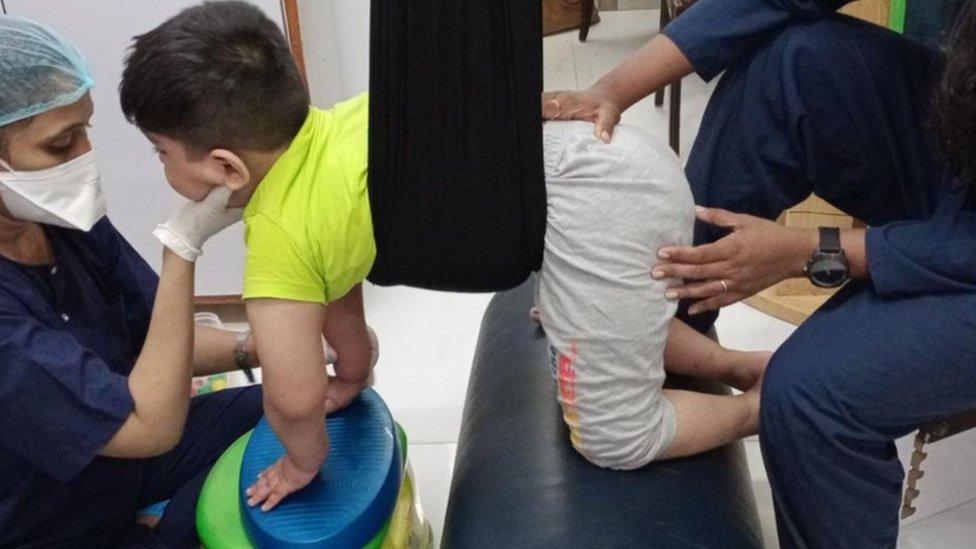
Affan, who is an SMA type-2 patient has to undergo physiotherapy every alternate day
While about 95% of rare diseases have no approved treatments, recent breakthroughs in medicine have made treatments available for genetic conditions like SMA and Duchenne Muscular Dystrophy (DMD) - which causes muscle weakness and degeneration. So Indians battling these diseases say they want to access the available drugs.
In March, over 100 parents whose children have DMD, external held a rally in capital Delhi to ask the government to bring new treatments to India, and to provide free medication and physiotherapy to all children with the disease.
India has a rare diseases policy which aims to provide financial help, but experts say it hasn't been implemented properly yet.
SMA is one of the better-known rare diseases in India because of some recent successful crowdfunding campaigns. The disease is caused by a defect in the SMN1 gene which leads to a deficit in a vital protein necessary for motor neurons to survive. SMA progresses over time, affecting a person's ability to walk, speak, eat and breathe.
According to Dr Sheffali Gulati, a child neurologist at the All India Institute of Medical Sciences in Delhi, there are four main types of SMA:
Type-1: the most severe and common version, where symptoms show up at birth or by the age of six months. Children have floppy limbs, don't reach motor milestones and most patients don't live beyond two years.
Type-2:has its onset between three and 15 months of age. Though these children can sit, they can't stand or walk on their own.
Type-3 and Type-4 are less severe, but in these cases too gradual muscle deterioration is imminent, shortening the person's lifespan.
"While SMA can't be cured, available treatments may slow down or stop the progression of the disease, prolonging a person's life and improving its quality," Dr Gulati says.
The US Food and Drug Administration has approved three treatments so far:
The most expensive is Zolgensma gene therapy at around 175m rupees for a one-time infusion that replaces the faulty SMN1 with a new functional one. Doctors and parents say a dose costs around 175m rupees in India excluding taxes; a spokesperson for Novartis, which makes the drug, said they couldn't comment on its price.
Spinraza costs about 50m rupees for the first four doses; after that the doses cost approximately 30m rupees a year. It has to be taken life-long.
A year's supply of Risdiplam, the cheapest of the lot, costs around 7.2m rupees. It has been approved for use in India and is sold here. This, too, has to be taken life-long.
None of these drugs are made in India and all of them are patented - so there are no cheaper generic alternatives available.

Alpana's son Aarav is an SMA type-2 patient
Some experts have criticised pharma companies for unreasonable pricing of drugs for rare diseases. But others argue that it helps manufacturers, external recoup the billions spent on research and development and fund future research.
Most patients in India access SMA drugs through humanitarian programmes offered by the drug manufacturers. These schemes offer free drugs to a limited number of patients, but parents say this doesn't always guarantee a lifetime supply.
And factors like age or health issues might render a person ineligible for these programmes. Novartis, which manufactures Zolgensma, offers the drug to children under two years through a lottery system.
Advocacy groups say there's scope to make Risdiplam cheaper in India. "We have been asking the government to strike a deal with the manufacturer," says Alpana Sharma, co-founder of CureSMA India, a parent-led advocacy group.
The foundation has also asked for the goods and services tax - 12% - on the drug to be removed and have approached the federal health ministry, finance ministry and top government doctors with these requests.
The BBC is awaiting a response to emails sent to the federal health and finance ministries.
CureSMA India has also petitioned the Delhi high court. In July, the court directed the National Rare Diseases Committee, external - an expert panel it set up to implement India's rare diseases policy - to speak to manufacturers to see if SMA medicines could be procured at lower prices.
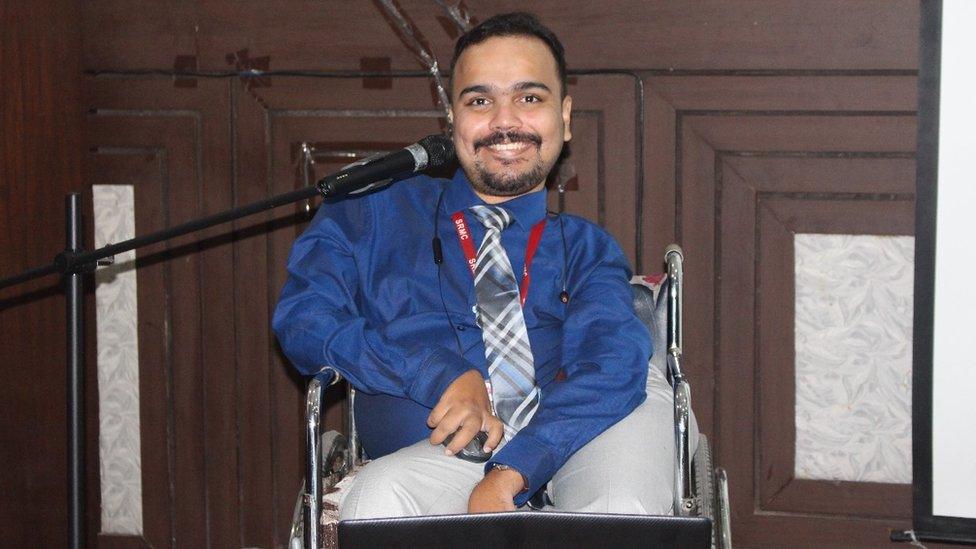
Abhinand was diagnosed with SMA type-3 at the age of two
These advocacy groups are looking to Brazil for hope - in December, the government said, external it would cover the cost of Zolgensma for infants with the most severe cases of SMA after dozens of families fought and won court cases. The Brazilian government has struck a confidential deal with Novartis to buy the drug at a reduced price and pay for it in instalments, the New York Times reported.
But the article adds that the policy has strained an already-struggling public health system and consumed a disproportionate share of resources.
Public policy experts such as Dr Chandrakant Lahariya say India may also face similar challenges. "Governments always have to think of the good of many over the good of a few. It's an ethically and economically difficult decision to make," he says.
Ms Sharma says that some parents and patients may be able to pull together funds to pay for SMA treatment, but not at the current prices.
Meanwhile, a lack of access to these life-saving drugs is putting people's lives on hold.
Abhinand, 35, an assistant professor of bioinformatics in Chennai city, was diagnosed with SMA type-3 at the age of two. He stopped walking at the age of 12 and his muscles have degenerated over time. He uses a wheelchair and has severe scoliosis which makes it difficult for him to breathe.
The recent availability of new treatments has given him some hope after years of struggle. In 2021, he raised enough money to afford a year's supply of Risdiplam - 30 bottles - through crowdfunding, and a relative agreed to sponsor his treatment for the next year. But after his medicines run out, Abhinand doesn't know what he will do as his health conditions make him ineligible for compassionate programs.
"I've been in a relationship with an amazing woman for the past seven years," he says.
"But I don't have the heart to ask her to marry me unless I'm sure I'll be able to get medication to survive. I just can't do that to her."
BBC News India is now on YouTube. Click here, external to subscribe and watch our documentaries, explainers and features.

Read more India stories from the BBC:

Related topics
- Published4 August 2022

- Published13 August 2022
