Coronavirus: Germany set to limit AstraZeneca jab to under-65s
- Published
- comments
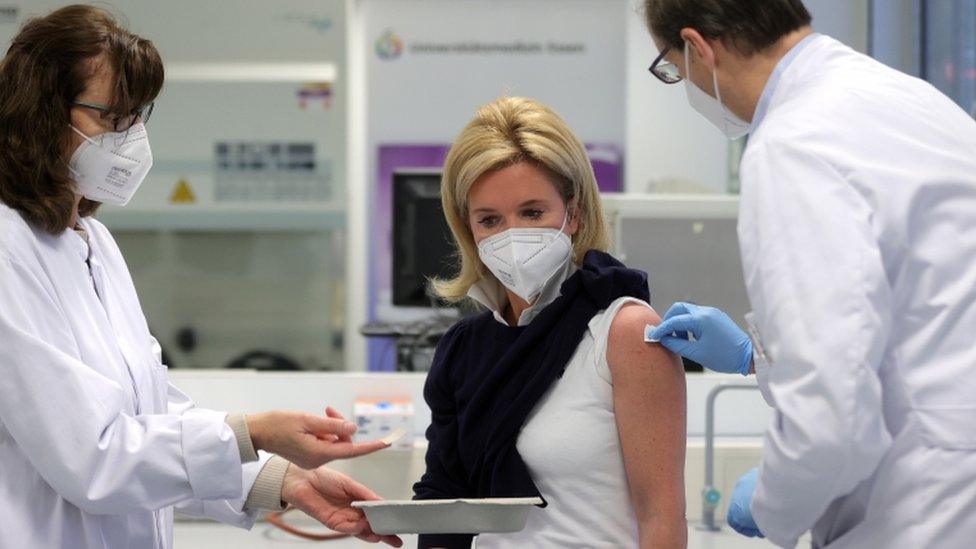
Germany's government has been criticised for its vaccine programme's slow start
Germany's vaccine committee has said AstraZeneca's Covid jab should only be given to people aged under 65.
The committee cited "insufficient data" over its efficacy for older people.
The European Medicines Agency is to decide on Friday whether to approve the vaccine for use across the EU.
The UK has been using the AstraZeneca vaccine in its mass immunisation programme for weeks now, and public health officials say it is safe and provides "high levels of protection".
The German announcement comes as the EU is in dispute with leading manufacturers over a shortage of vaccines on the continent.
UK-based AstraZeneca has said production issues at its Europe-based plants mean it will be unable to deliver the promised number of doses to the bloc.
But the EU says the firm must honour its commitments and deliver the jabs by diverting stock from the UK. It is calling on members to "make use of all legal means" to secure the supply.
Pfizer-BioNTech has also cut the number of doses it is delivering to the 27-member bloc.
UK confident 'safe' vaccine has 'substantial benefits'
The independent vaccine commission advising the German government said there were "currently insufficient data available to assess the vaccine efficacy from 65 years of age" and recommended "the AstraZeneca vaccine... should only be offered to people aged 18-64 years at each stage".
But Dr Mary Ramsay, Head of Immunisations at Public Health England, said both the AstraZeneca and Pfizer-BioNTech vaccines are "safe and provide high levels of protection against Covid-19.
"There were too few cases in older people in the AstraZeneca trials to observe precise levels of protection in this group, but data on immune responses were very reassuring."
UK Prime Minister Boris Johnson said he was not worried by the German recommendation, saying British health authorities had made clear the AstraZeneca vaccine was "effective across all age groups".
Meanwhile, Paul Hunter, professor of Medicine at the University of East Anglia, told BBC News that the jab was safe for people over 65 "almost certainly provides substantial benefits in terms of preventing severe disease and reduce the chances of going into hospital".


All of the regulators and experts in different countries have been looking at the same data on the Oxford-AstraZeneca vaccine. That data comes from clinical trials, and those did recruit fewer elderly people overall.
That's because they started off first with younger volunteers to get results as quickly as possible, given the urgency to find out if a vaccine would work well enough to help get us out of the pandemic.
The scientists who ran the trials have always been upfront about this. But they say there is other evidence to suggest the vaccine will work well in older adults. Studies show the over 65s have a strong immune responses to the vaccine. After receiving the shots their blood has plenty of the required antibodies that can fight coronavirus.
The UK has been using the AstraZeneca vaccine in its mass immunisation programme for weeks now and should soon have more proof from the real world setting about how much protection the shots give.

What's happening with the rollout in the EU?
The pace of the EU's vaccination programme has been criticised in recent weeks.
There are shortages due to delays in shipments of both the Pfizer-BioNTech and Moderna vaccines, which are the only ones currently approved for use in the block.
Vaccination appointments are being delayed in many countries. In Madrid, Spain, authorities have suspended first doses of the vaccination for at least two weeks.


Sweden was set to receive one million doses of the AstraZeneca vaccine in February but is now expected to get just 400,000 doses.
European Council President Charles Michel commented on AstraZeneca's inability to provide all the doses expected over the coming weeks.
"If no satisfactory solution can be found, I believe we should explore all options and make use of all legal means and enforcement measures at our disposal," he said in a letter to EU leaders.

Toned down rhetoric

On Thursday the EU tried to dial down the emotion in its ongoing battle to get AstraZeneca to deliver the number of vaccines stipulated in their contract. Until now Brussels had reacted with outrage at the news it might only get a fraction of the vaccines expected from Astra Zeneca.
Now it's toning down the rhetoric. Instead the president of the European Council threatened legal action. The EU has also stepped away from involving the UK in the row.
It is still arguing that AstraZeneca should use its plants in the UK, as well as those in the EU, to supply the bloc with promised vaccines. Brussels also disagrees with the UK government that its contract with AstraZeneca should get priority as it was signed first.
But in public, the European Commission was keen to underline today that its dispute was with AstraZeneca, not the UK government.

What's behind the supply problems?
The EU signed a deal with AstraZeneca in August for 300 million doses, with an option for 100 million more, but the UK-Swedish company has reported production delays at plants in the Netherlands and Belgium.
AstraZeneca's CEO said production was "basically two months behind where we wanted to be".
Stella Kyriakides: "AstraZeneca needs to deliver on its commitments"
The EU had hoped that as soon as approval was given - which is expected on Friday - delivery would start straight away, but the production issues have dented this hope.
Reports said last week the EU would get 60% fewer vaccine doses - about 50 million jabs - than promised in the first quarter of the year.
The bloc is also facing delays with supplies of the Pfizer-BioNTech vaccine, and it has a much bigger deal with the US-German vaccine maker.
Both the EU and AstraZeneca have vowed to work together to resolve the problems.
- Published28 January 2021

- Published23 January 2021
- Published22 January 2021