Coronavirus: What's behind France's AstraZeneca turnaround?
- Published

President Macron earlier expressed scepticism of the vaccine's effectiveness among older people
It was just a month ago that French President Emmanuel Macron said the Oxford-AstraZeneca vaccine was "almost ineffective" in the over-65s.
His Europe minister then accused the UK of taking "massive risks" by depending too heavily on its home-grown jab.
Mr Macron has now said that he would happily receive the AstraZeneca vaccine if that was what he was offered. His government also announced an apparent U-turn by approving the jab for some older people.
So what has changed? Have the French now seen the limits of criticising AstraZeneca and come to understand that there can be no such thing as too many vaccines?
Or have the UK's accusations of French vaccine nationalism been wide of the mark? After all, it's not a race, and if Europeans chose to be careful over the AstraZeneca vaccine because they felt there was a lack of clinical evidence for the over-65s, that would hardly be the first time the EU's precautionary principle has slowed things down.
The truth is probably a mixture of the two.
On the one hand, it is hard not to believe that there was some element of post-Brexit ill will motivating the French and wider European scepticism towards the AstraZeneca jab.
But it was noticeable how the remarks made by Mr Macron and Europe Minister Clément Beaune that cast doubt on the vaccine's effectiveness never found a receptive audience in the French medical profession.
That profession - which had no political axe to grind - said early on that the jab was a welcome addition to the mix.
But the politicians set the tone. They must surely take some of the blame for the slow uptake of the vaccine since its launch in France last month.
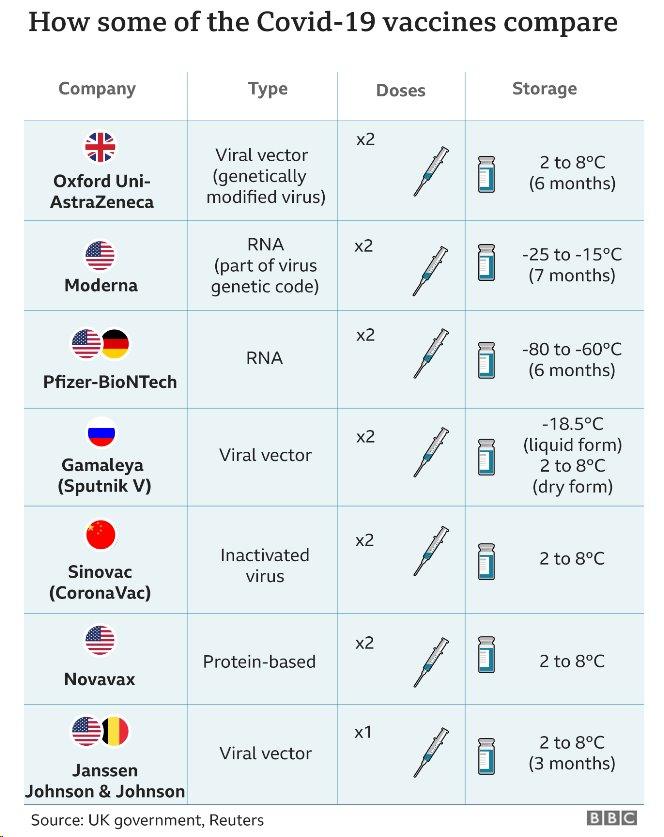
WATCH: Pfizer v Oxford v Moderna – three Covid-19 vaccines compared
Only a quarter of the AstraZeneca doses delivered to France have actually been put into people's arms.
That can partly be attributed to the different method of distribution compared to the more widely-used Pfizer jab. For the AstraZeneca jab, doctors are waiting for people to come to their surgeries while for Pfizer there has been a more accessible market in hospitals and care homes.
Nonetheless, the government's own vaccination co-ordinator Alain Fischer has admitted that the AstraZeneca vaccine has had "a pretty bad press in France". This is a clear sign that the messaging surrounding it has been far from helpful.
AstraZeneca has come to be seen by too many people here as an inferior vaccination - partly because of its lower level of protection compared to the other jabs, and partly because of widespread reports of mild side-effects.
The French government, however, now seems bent on changing that perception and accelerating the take-up of AstraZeneca. That is why it has now approved its use for at-risk over-65s.
There have been accusations of opportunism, with some suggesting that the French government wants doses of AstraZeneca merely to save its struggling vaccination programme. But there is in fact a perfectly sound response.
France never ruled out using the jab on older people; it just said it was waiting for evidence that it worked. And now - with the new Scottish study - that evidence seems to be there.


- Published2 March 2021
- Published29 January 2021
- Published28 February 2021