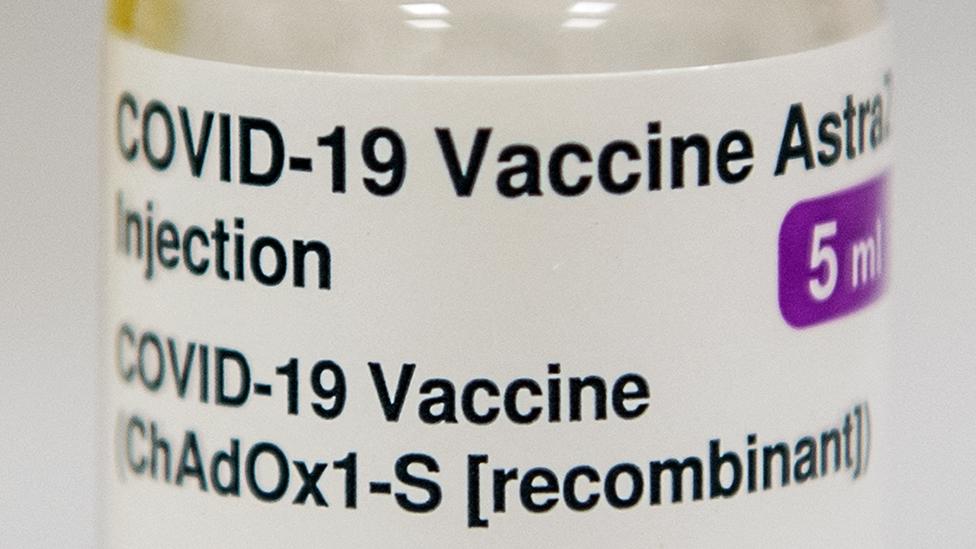Covid: France approves AstraZeneca vaccine for over-65s
- Published

The French government says older people with pre-existing conditions can now get AstraZeneca's Covid-19 vaccine, revising its stance on the issue.
"People affected by co-morbidities can be vaccinated with AstraZeneca, including those aged between 65 and 74," the health minister said.
Last month France approved use of the vaccine for under-65s only, citing lack of data for older people.
Since then studies have shown the jab is highly effective among the elderly.
The Oxford-AstraZeneca vaccine is widely used across the UK, but several EU countries are still limiting it to the under-65s, including Germany.
The EU drugs regulator has approved it for all adults, but it is up to each member to set its own roll-out policy.
In a further development, Canada's immunisation commission on Monday advised against giving the AstraZeneca vaccine to over-65s, saying clinical trial data for that age group was too limited.
What are the French saying?
Speaking on television, French Health Minister Olivier Véran said people with pre-existing conditions - such as high blood pressure or diabetes - could get the Oxford-AstraZeneca vaccine from GP surgeries, hospitals and "within days" from pharmacies.
The policy would apply to those over 50, including those aged 65 to 75, he said.
Those aged over 75 will still be offered either Pfizer or Moderna jabs in a vaccination centre, he added.

Studies have shown the AstraZeneca vaccine to be safe and effective
In January French President Emmanuel Macron said the AstraZeneca vaccine was "quasi-ineffective" for older age groups - a claim strongly rejected at the time by the UK officials and scientists.
But after a European Council meeting on Friday, he said: "If this is the vaccine I'm offered, obviously I would take it."
As more data has emerged, French health officials have tried to convince people that it is just as safe and effective as other Covid-19 vaccines.
Just 273,000 AstraZeneca doses have been administered in France out of 1.7 million received by the end of February, health ministry figures show.
Some French doctors had spurned the vaccine, citing initial side-effects in some people and trial data suggesting it offered minimal protection against mild disease from the South African Covid variant, though the developer said it still protects against severe disease.
The MG France doctors' association has since hit back at criticism of the AstraZeneca jab and the fact that many doses remain unused. The man in charge of France's vaccine rollout has also backed it, saying it has unfairly received a "bad press".
About three million people have so far received at least one dose of a Covid-19 vaccine in France - against more than 20 million in the UK, which has roughly the same population.

Change of tone

It is hard not to believe that there was some element of post-Brexit ill will motivating the initial French scepticism towards the AstraZeneca jab.
But it was noticeable how the remarks made by Mr Macron and Europe Minister Clément Beaune that casted doubt on the vaccine's effectiveness never found a receptive audience in the French medical profession.
That profession - which had no political axe to grind - said early on that the jab was a welcome addition to the mix. But the politicians set the tone. They must surely take some of the blame for the slow uptake of the vaccine since its launch in France last month.
AstraZeneca has come to be seen by too many people here as an inferior vaccination. The French government, however, now seems bent on changing that perception and accelerating take-up.

France is still struggling to control increased infection rates in some areas, despite a continuing national night-time curfew.
Bars, restaurants and museums remain closed but the government wants to avoid another national lockdown, resorting instead to tighter measures in some localities such as the southern city of Nice.
Travel to Germany from the French Moselle region has been restricted at the request of Germany which is concerned about the South African variant. Cross-border public transport is suspended, while drivers can cross but only with proof of a negative coronavirus test.
What is the situation in Germany?
Germany is also concerned that AstraZeneca jabs are going to waste and there are calls to widen the number of priority groups who can receive it. Only 240,000 of 1.45 million doses had been used by 23 February.
On Sunday, a senior German immunologist, Carsten Watzl, urged his country to change its mind and start allowing over-65s to receive the vaccine.
Germany's vaccine commission is currently reviewing its recommendation and Chancellor Angela Merkel said last week that it was "a vaccine that can be trusted".
Covax vaccine plan: What is it and how will it work?
Vaccine roll-outs in many EU countries have been hit by delays.
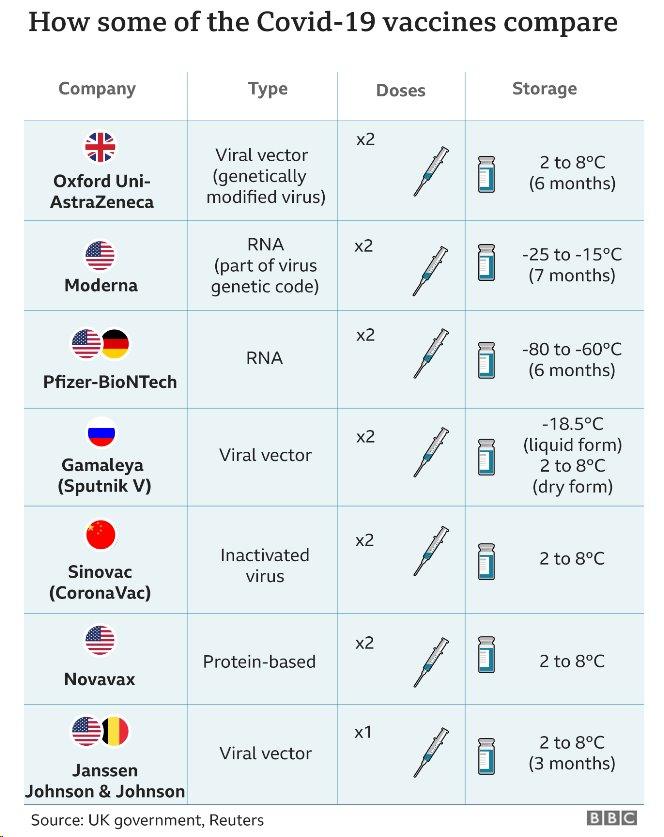
What do we know about the vaccine?
Britain has been using the vaccine made by AstraZeneca, a UK-Swedish pharmaceutical firm, in its mass immunisation programme since December.
UK health officials say it provides "high levels of protection" for all ages.
No one who received the Oxford vaccine in trials was admitted to hospital or became seriously ill due to Covid.
The vaccine is given via two injections to the arm, the second between 4 and 12 weeks after the first.


Related topics
- Published28 February 2021
- Published29 January 2021
- Published7 May 2021