Covid: Brazil allows resumption of Chinese vaccine trial
- Published
Anvisa said it now had "sufficient information to allow vaccination to resume"
Brazil's health regulator has announced that the trial of a Chinese coronavirus vaccine can resume.
Anvisa suspended Brazil's trial of the vaccine two days ago, citing a "severe adverse incident" - reported to be the death of a volunteer.
The head of the institute conducting the trial said the death had no connection to the vaccine.
In a statement on Wednesday, Anvisa said it now had "sufficient information to allow vaccination to resume".
"It is important to clarify that a suspension does not necessarily mean that the product under investigation does not offer quality, safety or efficacy," it said.
President Jair Bolsonaro earlier declared the suspension a "victory".
He has long criticised the vaccine because of its Chinese links and said it would not be purchased by his country. He has also engaged in a political fight with the governor of São Paulo, João Doria, who has publicly backed the trial.
Mr Bolsonaro has not yet commented on Anvisa's announcement that the trial can resume.
The vaccine, developed by Chinese firm Sinovac Biotech, is one of several in final-stage testing globally. Sinovac says it is "confident in the safety of the vaccine".
Brazil has been one of the countries worst affected by coronavirus, recording more than 5.6m confirmed cases - the third highest tally in the world after the US and India - and nearly 163,000 deaths, according to data collated by Johns Hopkins University.
Why was the trial halted?
On Monday, Anvisa said it had "ruled to interrupt the clinical trial of the CoronaVac vaccine after a serious adverse incident".
It did not reveal what had happened, nor where it had taken place.
Dimas Covas, head of the Butantan institute conducting the trials, told local media that the trial's suspension was related to a death, but insisted that the death was not linked to the vaccine.
This was backed up by Jean Gorinchteyn, health secretary for the state of São Paulo, who told a news conference that the death was an "external event" that was not related to the vaccine.
Mr Covas said that there had been no adverse reactions to the vaccine, and that the decision to suspend the trial had caused "indignation".
Media reports say police are investigating the death as a suicide.
In its statement allowing the resumption of the trial on Wednesday, Anvisa said it now had more details on the nature of the "adverse incident".
It said its decision to suspend the trial "took into consideration the data known to the agency at the time".
A pause in a clinical trial is not unusual. In September, the UK paused trials for another Covid-19 vaccine after a participant had a suspected adverse reaction.
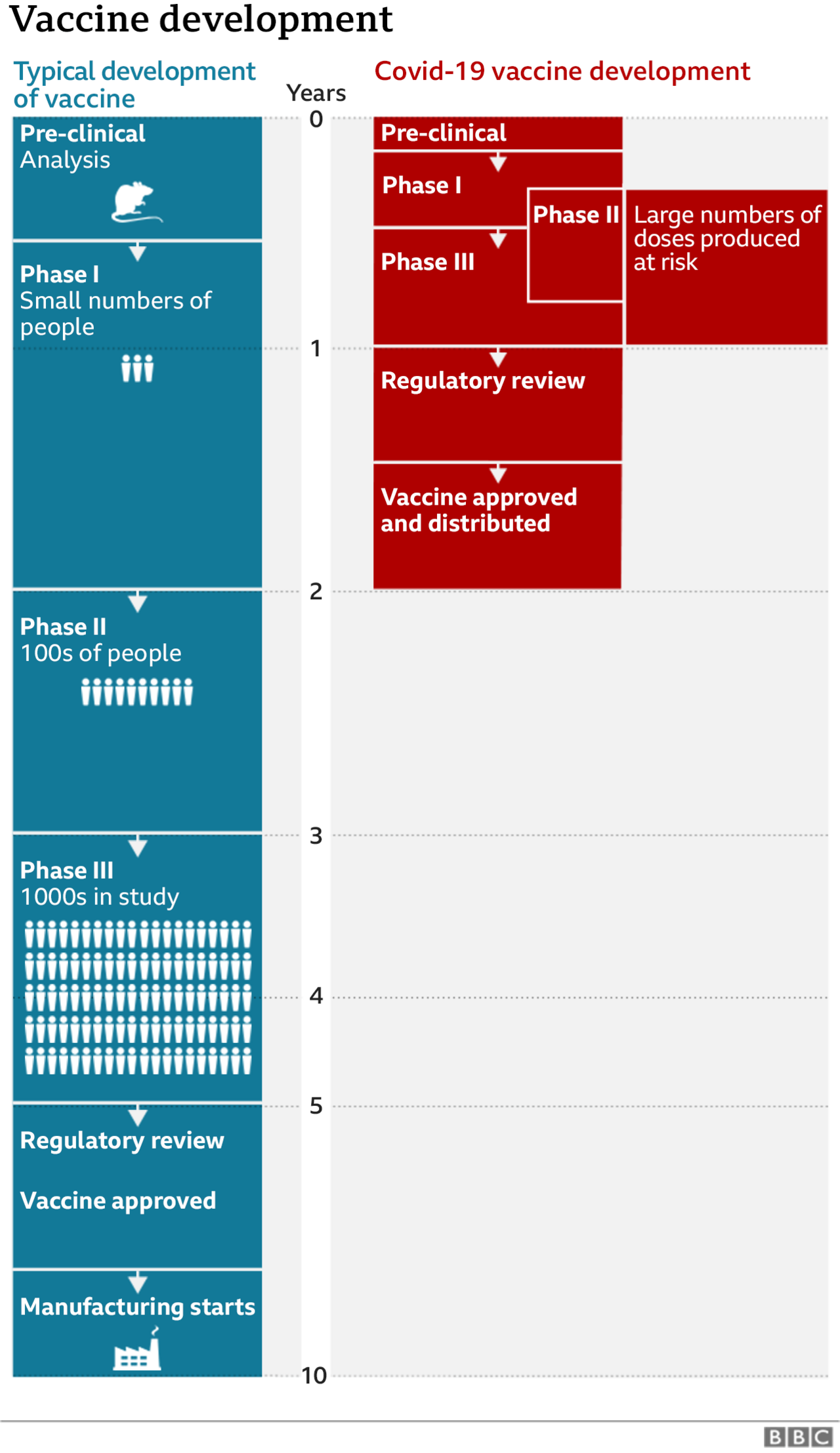
The trials for the vaccine being developed by AstraZeneca and Oxford University were resumed a few days later after regulators said it was safe to continue.
In October, a review into the death of a Brazilian volunteer involved in the AstraZeneca trial found no safety concerns. The BBC understands the volunteer did not receive the vaccine.
Brazil's president has been open about his preference for the vaccine being developed by AstraZeneca, saying his government would not buy a Chinese-made Covid-19 vaccine.
- Published13 August 2020
- Published17 October 2020

- Published12 September 2020

- Published21 October 2020
- Published27 August 2020
