Covid vaccine: When will Americans be vaccinated?
- Published
The day the US began Covid vaccinations
As health workers begin receiving Covid-19 vaccinations across the US, many Americans are asking when they will be able to get their shots.
It's complicated, but this is what we know.
Americans who are under 65 and healthy may be able to get their vaccinations as early as April, says Dr Anthony Fauci, who heads the National Institutes of Allergy and Infectious Diseases.
The Trump administration's health secretary, Alex Azar, predicts that by "late February, into March," Americans will be able to get vaccinated at pharmacy and grocery chains like CVS and Walmart.
But cases in the US are continuing to spike as winter sends people indoors and the holidays bring families together.
More than 300,000 Americans have died and 16.5 million have been infected since January 2020, and the US faces an uphill battle the curb the virus.
Here's what you need to know about getting vaccinated in the US.
Who is getting the vaccine first?
Centers for Disease Control and Prevention (CDC) guidelines submitted to US states say that the nation's 21 million healthcare workers should be prioritised first, as well the three million elderly Americans living in long-term care homes.
Approximately 87 million essential workers are expected to be next in line for the jab, but it will be up to states to decide which industries to prioritise.
Moncef Slaoui, the chief scientist of federal vaccine distribution programme Operation Warp Speed, says the young and healthy should be last in the line.
At least 70% or 80% of the US population of 330 million have to be vaccinated in order to achieve herd immunity, he warned on Monday, external.
Officials say vaccinations for groups that are not at a high risk are expected to take place in the spring of 2021.
What is the status of the vaccines?
One of the main challenges with vaccinating the US population is producing and distributing the shots.
Vaccinations in the US began on 14 December, with a critical care nurse in New York City receiving the first injection of a drug developed by Pfizer and BioNTech.
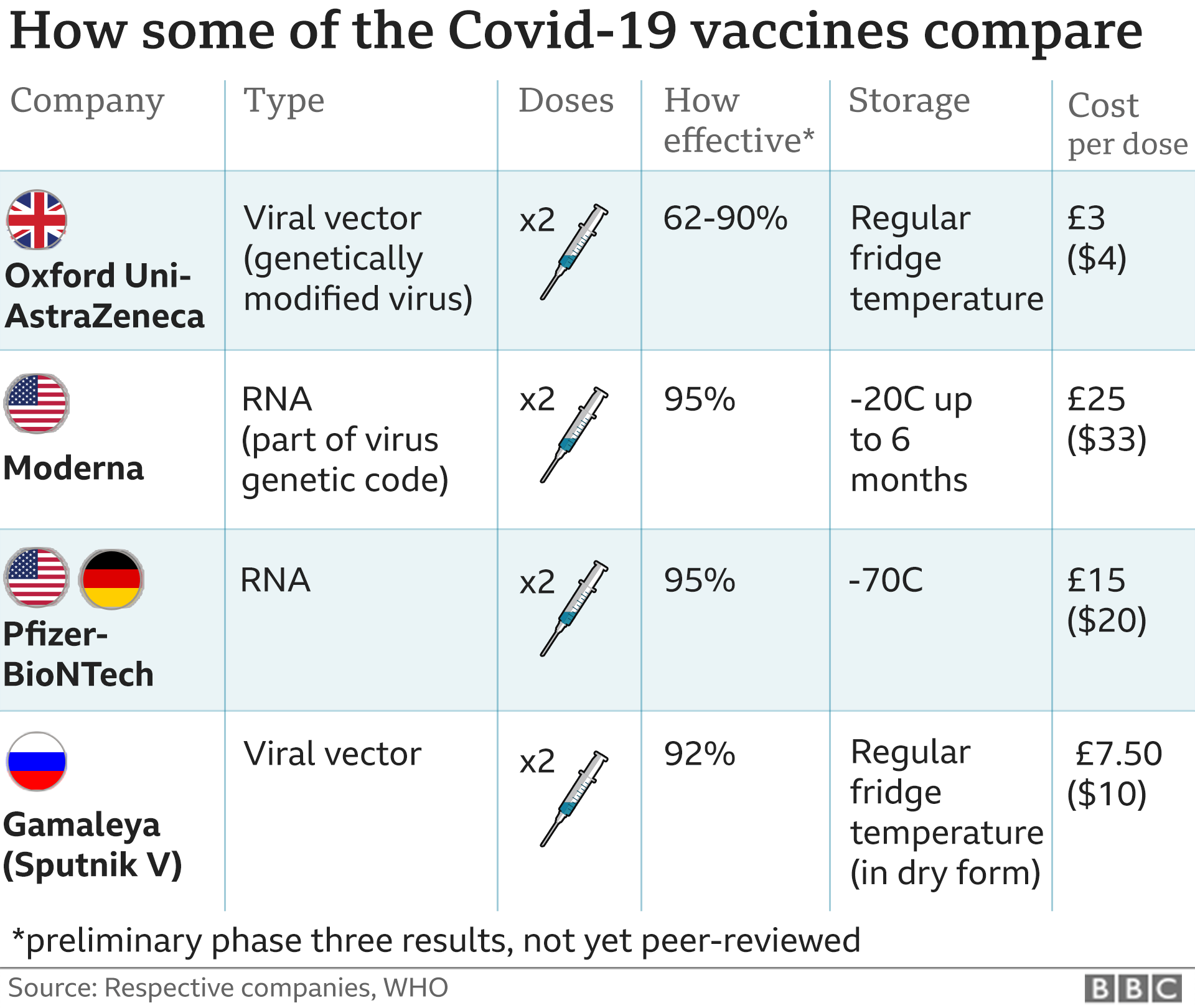
Pfizer, based in New York, and German company BioNTech received emergency authorisation from the Food and Drug Administration (FDA) after data found their vaccine 95% effective in preventing infections.
On Tuesday, the FDA reported that Moderna, a US company seeking approval for its vaccine, produced a drug that is 94% effective in warding off the virus. The rollout of this drug could begin early next week.
The FDA's Emergency Use Authorization (EUA) allows a drug to be used as a treatment while studies are still being carried out to determine safety and effectiveness. Critics say the process is less rigorous and more likely to lead to health complications.
Johnson & Johnson, another US drug company, is currently testing a vaccine and could have enough data to submit an EUA request by January.

Five challenges of distributing a Covid-19 vaccine around the world.
How much vaccine will the US get?
Pfizer's first shipment of vaccine to the US saw 2.9 million doses sent to multiple locations around the country.
The company says they hope to have enough vaccine for 25 million Americans produced by the end of the year, and enough for 50 million by March.
The US has agreed to buy 200 million doses of vaccine from Moderna, a company that has never previously had a product approved by the FDA.
If approved, US officials say the Massachusetts-based company will immediately ship around six million doses to 3,285 sites across the US - far more locations than Pfizer's initial rollout.
Both vaccines require a second round of injections. Some pharmacies have been tapped to use their electronic networks to alert patients when it is time to receive their second shot.
For Pfizer, the second jab comes three weeks after the first. For Moderna it is four weeks after the first shot.
Johnson & Johnson's experimental vaccine is the only one being developed that relies on a single jab.
How will the new Pfizer vaccine work?
When will life return to normal?
Even as distribution begins, the drug manufacturers must continue to seek full - rather than emergency - approval, as the FDA continues to monitor the vaccines for any side-effects.
Health experts say the vaccine's arrival does not mean life will immediately return to normal.
"A vaccine will complement the other tools we have, not replace them," said Tedros Ghebreyesus, the head of the World Health Organization (WHO).
"A vaccine on its own will not end the pandemic."
Hand-washing, social distancing and mask-wearing will remain the norm until enough of the population has been vaccinated.
Dr Fauci says Americans may be able to roll back those necessary precautions by the autumn of 2021 if 70% of the US population has been vaccinated by then.
Will enough Americans trust it?
There are ongoing concerns regarding how many Americans are willing to get vaccinated. A recent Gallup poll, external found that 58% say they would be willing to get the jab, up from a low of 50% in September.
It also remains to be seen how racial minorities - who are at a higher risk of catching the virus and dying from it - will be prioritised and what level of trust they will have in the government-issued vaccine.
Former US presidents Barack Obama, George W Bush and Bill Clinton have vowed to be vaccinated on camera, in an effort to encourage others to get the jab. President Donald Trump says officials at the White House - which has seen several outbreaks - "should receive the vaccine somewhat later... unless specifically necessary".
Could states make the vaccine mandatory?
According to a 1905 landmark Supreme Court case, states do have it in their power to require the vaccination of their residents.
The case, Jacobson v Massachusetts, arose during a smallpox epidemic in Cambridge, Massachusetts, in 1902 when one resident refused the vaccine.
At the time, Cambridge said all residents must get vaccinated or re-vaccinated, otherwise pay a $5 fine (which would be about $150 in 2020).
In its ruling, the Supreme Court said that the rights of the individual do not trump the need to ensure public safety.
However, the high court of Massachusetts later ruled that states lacked the authority to administer the injection, and could only issue fines.
Pennsylvania's health secretary has said they will not issue any mandate, but Virginia's top health official has said they will consider issuing one once the vaccines are ready.
Can companies order employees take the vaccine?
Private companies in the US have the right to force their employees to get certain vaccines, experts say, but are unlikely to immediately do so once the vaccine is approved.
This is because the vaccines are both seeking approval under an emergency process that critics say could lead to health complications.
"Companies could theoretically issue a mandate, but in the current political climate it is very unlikely they will do so," George Washington University Law professor Peter Meyers told Reuters. "Americans tend to shy away from mandates."
Ford Motor Co and Kellogg Co have both said they will make the vaccine available to their employees on a voluntary basis.
Many hospitals and health clinics require annual flu shots, and all 50 states have vaccination requirements in place for school children.
Workers can be excused from a vaccine if they have health issues that forbid receiving a vaccine, or - in some states - if they have religious objections.
What will the vaccines cost?
The CDC says that vaccines purchased with taxpayer money will free, but providers may still charge for administering the jab.
That fee may be reimbursed by health insurance companies or the Medicaid and Medicare programmes - social safety nets for low income and elderly Americans.
In July, the US Department of Health and Human Services announced a $1.95bn (£1.45bn) deal to secure 100 million vaccine doses from Pfizer. The agreement also allows the US government to purchase an additional 500 million doses.
Moderna received nearly $1bn from the US government for coronavirus research. The company is set to receive an additional $1.5bn for 100 million doses, according to a deal signed in August.
Billions of dollars have also been promised to other drug companies in the event that they are able to bring additional vaccines to market.
States are also racing to acquire ultra-cold refrigerators that are capable of storing the Pfizer vaccine, which must be kept at temperatures of -70C (-94 F) and is being shipped in packages of dry ice.
- Published14 December 2020
- Published9 December 2020

- Published30 November 2020
- Published9 December 2020
- Published8 December 2020

- Published8 December 2020