Mifepristone: 12 US states sue to expand access to abortion pill
- Published
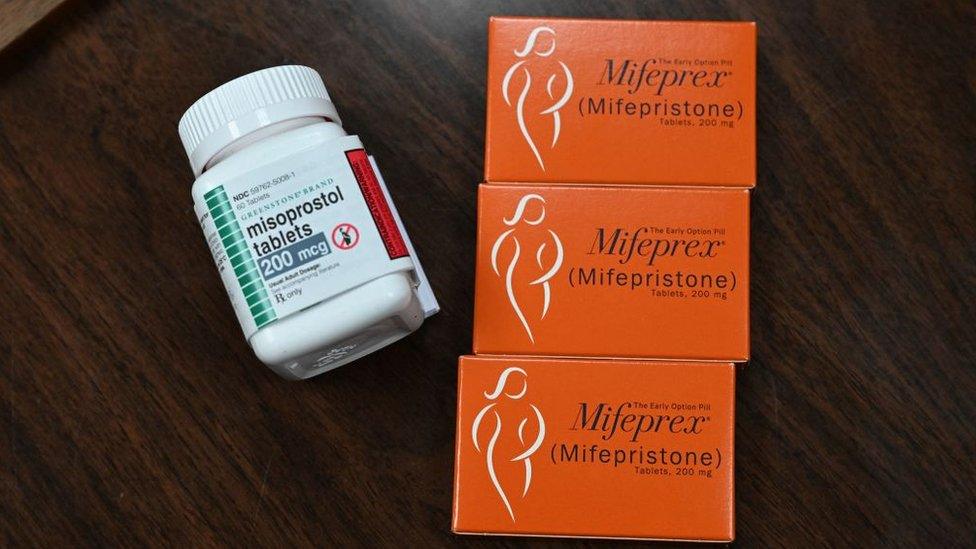
Medication abortion is the most common method of terminating a pregnancy in the US
A group of 12 Democratic-led US states has argued that the Food and Drug Administration (FDA) is hampering access to a popular abortion pill.
Mifepristone, part of a two-drug regimen that induces abortions, was approved in the year 2000, with restrictions to assure its safe use.
In a lawsuit filed on Thursday, the states claimed the limits on the drug are not supported by evidence.
Medication abortion is the most common method of the procedure in the US.
Now accounting for more than half of all abortions in the country, it has become the focus of growing political attacks since the US Supreme Court overturned the nationwide right to an abortion last summer.
In November, a coalition of conservative anti-abortion activists filed its own lawsuit, challenging the drug's approval.
The combination of mifepristone and another drug, misoprostol, is considered safe and highly effective in terminating pregnancies within the first 10 weeks.
But while misoprostol is freely available, the FDA tightly controls who can prescribe and dispense mifepristone.
That has created "burdensome restrictions" on a drug that is the "gold standard" for abortions and has a high safety profile, the Democratic suit argues.
Filed in federal court, it represents the states of Washington, Oregon, Arizona, Colorado, Connecticut, Delaware, Illinois, Michigan, Nevada, New Mexico, Rhode Island and Vermont.
"The availability of medication abortion has never been more important," wrote the plaintiffs, noting that approval of the drug was "based on a thorough and comprehensive review of the scientific evidence".
But restrictions on the drug have made it "harder for doctors to prescribe, harder for pharmacies to fill, harder for patients to access, and more burdensome... to dispense", they said.
Late last year, abortion opponents argued in their own suit that the drug's stringent regulation is evidence that it is dangerous to women and young girls.
The Alliance Defending Freedom legal advocacy group alleged that the FDA had overstepped its authority, approving the drug "without basis" and spending the next two decades "removing what few protections were initially in place".
Its suit calls on the court to order the FDA to withdraw mifepristone from the market entirely.
Related topics
- Published25 August 2022

- Published4 January 2023

- Published12 July 2022