Texas judge considers revoking FDA approval of abortion pill in US
- Published

Critics of the lawsuit have begun to gather outside the Amarillo courthouse
A Texas judge has heard arguments about whether a commonly used abortion pill should be sold in the US, in a ruling that could curtail access to the drug nationwide.
The pill, mifepristone, can be taken at home and is used in more than half of US pregnancy terminations.
A lawsuit filed by an anti-abortion group in Texas argues that the drug's safety was never properly studied.
Mifepristone has been approved for use for over 20 years.
The case, which will be decided by Judge Matthew Kacsmaryk, an appointee of former President Donald Trump, follows the US Supreme Court's historic overruling last year of the constitutional right to abortion.
After Wednesday's four-hour hearing, he said he would issue his ruling as soon as possible.
The Texas lawsuit, filed by the Alliance for Hippocratic medicine, an anti-abortion organisation, argues there are three reasons why the drug should be be removed from shelves.
One, the lawsuit says the US Food and Drug Administration (FDA) erred by approving the pill under a clause intended to fast-track drugs used for life-threatening illnesses.
Two, it argues that the FDA approved mifepristone before adequate testing was carried out.
Three, it argues that it is illegal to ship the drug under the Comstock Act, an 1873 law that banned the posting of abortion drugs. However, last December the US Department of Justice's Office of Legal Counsel issued an opinion that the Comstock Act does not prevent the mailing of abortion medication intended for legal use.
In court, Judge Kacsmaryk described the FDA's approval process as accelerated, according to the Washington Post.
The FDA spent four years reviewing the drug before it was approved in 2000.
President Joe Biden's administration has responded to the lawsuit, arguing that mifepristone's approval was well supported by science.
The FDA has reported a total of 26 deaths associated with the drug since it was approved in 2000 - a rate of about 0.65 deaths per 100,000 by-pill abortions.
For comparison, the death rate associated with habitual aspirin use is about 15.3 deaths per 100,000 aspirin users.
If Judge Kacsmaryk rules that the FDA erred in its approval, sales of the drug - one of only two pills used to induce an abortion - could be halted.
Such an unprecedented ruling would essentially upend the entire foundation of America's independent drug regulatory system, says I. Glenn Cohen, a legal professor at Harvard University.
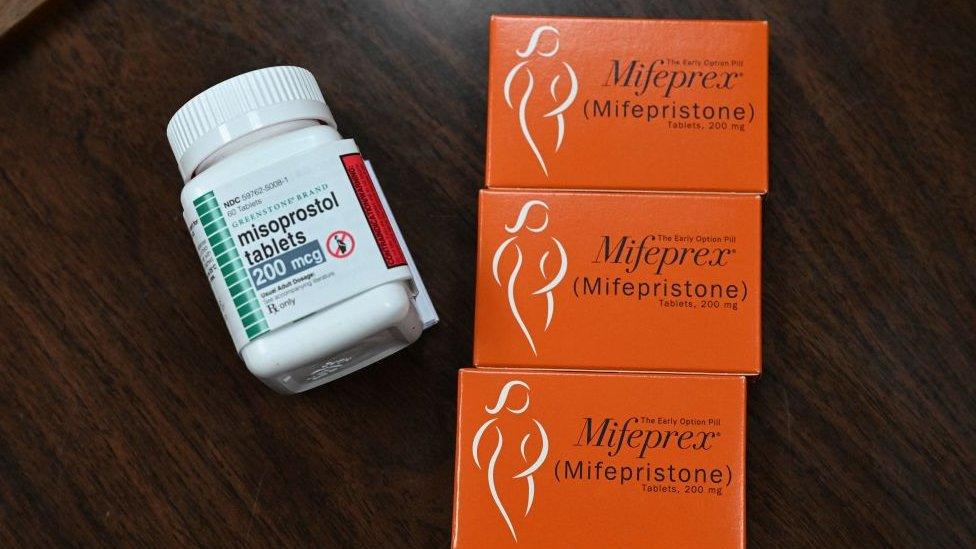
The lawsuit claims that the FDA approved mifepristone prematurely.
That could affect not only access to the drug for millions of women, but it could cause a chilling effect on drug companies developing new drugs, Prof Cohen said.
He said it was possible the case could go all the way to the Supreme Court.
If the drug was removed from shelves, women would still be able to use the other approved abortion drug, misoprostol.
Misoprostol can be used alone and is often the only option in countries where mifepristone is banned for abortions. Some US clinics and providers also only offer misoprostol.
The treatment, however, is slightly less effective than a two-drug regimen that also includes mifepristone.
Citing death threats and harassing phone calls, Judge Kacsmaryk had urged attorneys not to publicise the date of Wednesday's hearing.
Some media outlets criticised the rare request, citing transparency concerns, and sent a letter to the court.
A small amount of protesters, who are against the lawsuit, gathered outside the federal court in Amarillo.
Julie Marie Blake, senior counsel for the Alliance Defending Freedom, a conservative group that backs the lawsuit, told the BBC's US partner CBS that the organisation is "confident that when any court looks at the law and looks at the science, it will realise that the FDA has completely failed its responsibility to protect women and girls".
Meanwhile, twelve Democratic-led states, including Washington, Arizona, Colorado, Delaware and Connecticut, have filed a separate lawsuit against the FDA seeking to make access to mifepristone easier.
That legal action alleges that the FDA's current regulations concerning the drug are "burdensome, harmful and unnecessary".
Related topics
- Published24 February 2023
- Published19 January 2023

- Published4 January 2023
