Climate change: Ocean acidification affects Arctic Ocean most
- Published
- comments

Scientists are warning that wildlife living in the Arctic Ocean could be at huge risk because of ocean acidification.
That's where the ocean becomes more acidic from absorbing carbon dioxide when there's too much in the atmosphere.
A recent study found that this process is happening much faster in the Arctic Ocean than any other body of water in the world.
Scientists were shocked by the results and say it could get worse over the next decade.

There are lots of different marine animals, fish and plants that live in the Arctic Ocean that could be affected by ocean acidification
Ocean acidification
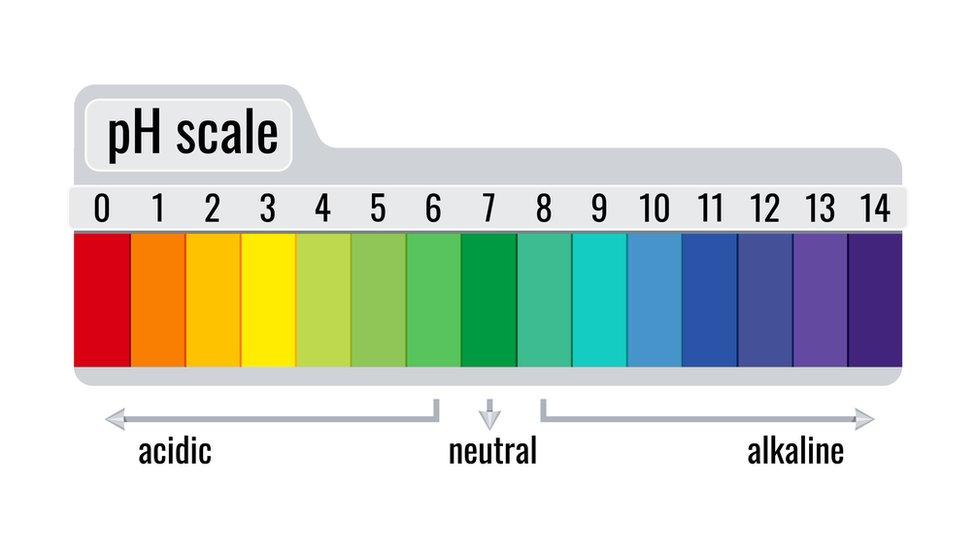
Seawater usually has a neutral pH - the scale of how acidic or alkaline a substance is.
The number 7 is considered neutral on the pH scale. Something is considered acidic if it has a pH which is less than 7 and alkaline if it's more than 7.
When there's too much carbon dioxide in the atmosphere, we know trees absorb some of this excess CO2, but so does the ocean.
In fact, the ocean absorbs a third of all the carbon dioxide in the atmosphere!
When carbon dioxide is absorbed, it increases the acidity of the liquid - in this case, the ocean.

The amount of carbon dioxide in our atmosphere has been steadily increasing since the Industrial Revolution
The amount of CO2 in our atmosphere has been steadily increasing since the industrial revolution when fossil fuels like coal and gas became widely used.
Fossil fuels produce carbon dioxide which is ultimately absorbed by our oceans.
We burn fossil fuels to produce energy.
Examples of fossil fuels include coal, oil and natural gases.
What has this new study shown?
Unlike other oceans, where the levels of ocean acidification are driven by an increase in carbon dioxide in the atmosphere due to fossil fuel, the Arctic Ocean has another problem to contend with - the ice is melting.
The Arctic Ocean - which is this Earth's northernmost body of water - is usually covered in ice but that's changing as temperatures continue to rise.
Scientists looked at data collected from the Arctic Ocean from 1994 to 2020 and compared it with the data from other oceans.

Scientists around the world have been studying the impact of climate change on the Arctic Ocean for years, but there's still lots more to look at!
They say they were shocked to find it is becoming more acidic up to four times faster than elsewhere.
When the sea ice melts, it soaks up even more carbon dioxide than it would normally because it's less able to resist the acidification process.
What consequences could this have?
Scientists are predicting it could have a big negative impact on wildlife in the sea.
There are lots of studies that shows that coral struggles to grow in water that is too acidic.
There are theories that it can make it difficult for animals to communicate under the water or even make metals more toxic.
But researchers don't have enough data yet to understand what other specific animals or systems could be affected.
- Published1 November 2021

- Published16 November 2021

