Flu drug given out "indiscriminately"
- Published
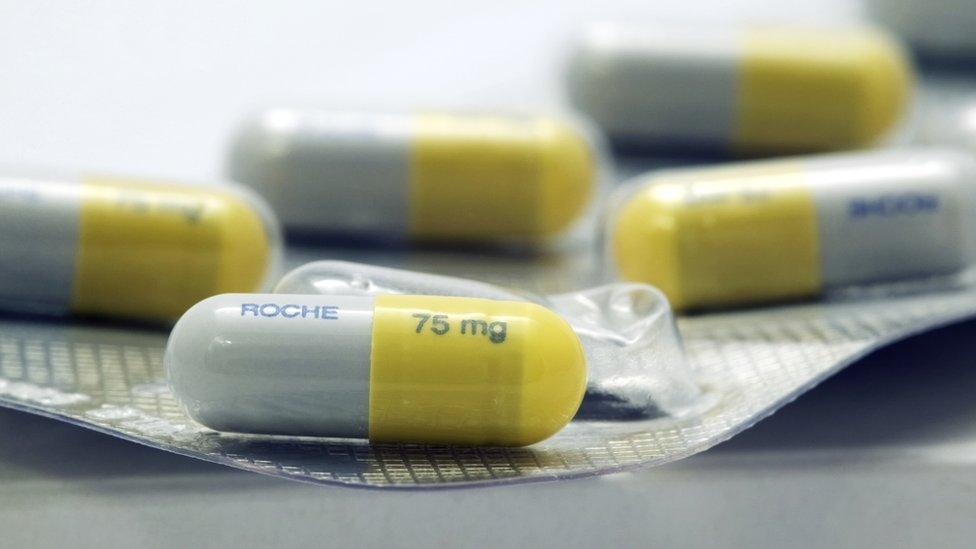
The antiviral drug Tamiflu was handed out "indiscriminately" during the last swine flu outbreak, a leading panel of UK scientists has said.
The experts said many thousands of patients had received treatment that may have done no good at all.
They are calling for comprehensive trials during the next pandemic, with some patients receiving the drug and others being given routine care.
The report was put together by the Academy of Medical Sciences.
Together with the Wellcome Trust the team reviewed all recent evidence on Tamiflu.
Their new analysis suggests the antiviral pills are helpful in certain, limited circumstances - for example for people unwell in hospital with seasonal flu.
But when it comes to pandemic flu, researchers say there needs to be much more work to find out if the drugs will provide a good defence.
They are urging hospitals and members of the public to be ready to take part in clinical trials when the next large flu outbreak emerges.
Prof Chris Butler, who was involved in the review, told the BBC: "Last time we gave people Tamiflu rather indiscriminately.
"We really missed a trick... by not doing clinical trials early on and just making assumptions.
"Here we are having treated many thousands of patients still not knowing whether it was a good thing or not."
Cautious approach
But experts say the approvals and infrastructure for these trials need to be put in place now, in "peace time", so they are ready to start when the next pandemic strikes.
Dr Butler added: "What we would like to happen next time is you would contact your GP practice and they would explain there is a trial going on.
"And you could then be randomised - that is allocated by chance - to get lots of fluids and paracetamol for example or that plus Tamiflu or another antiviral."
Prof Wendy Barclay, from Imperial College London, said the report was helpful in pulling together current knowledge about the drugs but warned there must be a cautious approach to trials so people are not denied treatment.
She said: "It will be important... to be clear about how such trials will be conducted, for example, how does one allocate a placebo (dummy pill) group in this situation?
"Whilst new styles of clinical trials are being designed to address these concerns, it is important that patients at high risk are not denied this licensed drug."
The report was developed after a request from the Department of Health. The expert group involved clinical researchers, industry and public health specialists.
- Published24 August 2015
- Published5 February 2015
- Published8 January 2015