Immunotherapy cancer drug hailed as 'game changer'
- Published

An immunotherapy drug has been described as a potential "game-changer" in promising results presented at the European Society for Medical Oncology congress.
In a study of head and neck cancer, more patients taking nivolumab survived for longer compared with those who were treated with chemotherapy.
In another study, combining nivolumab with another drug shrank tumours in advanced kidney cancer patients.
Immunotherapy works by harnessing the immune system to destroy cancer cells.
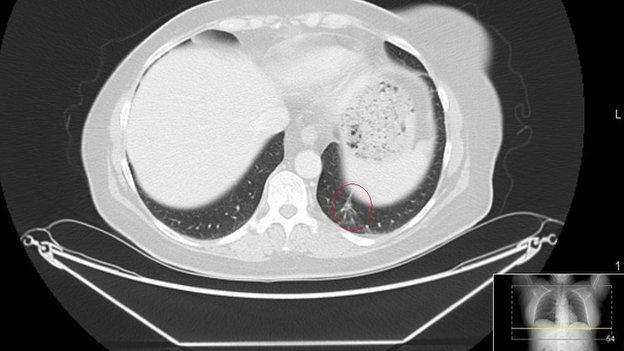
Advanced head and neck cancer has very poor survival rates.
In a trial of more than 350 patients, published in the New England Journal of Medicine, 36% treated with the immunotherapy drug nivolumab were alive after one year compared with 17% who received chemotherapy.
Patients also experienced fewer side effects from immunotherapy.
Double hit
The benefits were more pronounced in patients whose tumours had tested positive for HPV (human papillomavirus). These patients survived an average of 9.1 months with nivolumab and 4.4 months with chemotherapy.
Normally, this group of patients, with advanced or treatment-resistant tumours, are expected to live less than six months.
Early data from a study of 94 patients with advanced kidney cancer showed that the double hit of nivolumab and ipilimumab resulted in a significant reduction in the size of tumours in 40% of patients.
Of these patients, one in 10 had no sign of cancer remaining.
This compares with 5% of patients showing tumour reduction after standard therapy.
About 12,000 people are diagnosed with kidney cancer in the UK each year and an average of 12 people die from the disease each day.


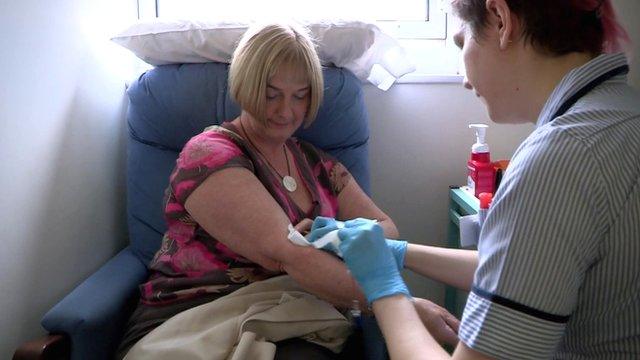
Peter Waite was able to continue working as a motor technician while receiving immunotherapy treatment for cancer
Peter's journey
"I feel a bit of a fraud having terminal cancer because I haven't been in pain at all," says Peter Waite, 64, from Hertfordshire.
"There's been nothing negative about it for me and I feel a bit embarrassed really."
Peter started receiving combined immunotherapy (nivolumab and ipilimumab) in a clinical trial in early 2015 after doctors discovered he had a type of renal cancer several years after recovering from kidney and lung cancer.
He was told he probably had three to five years left.
Instead of being treated with chemotherapy, he spent four months receiving both immunotherapy drugs and experienced virtually no side effects, allowing him to continue working as a motor technician throughout his treatment.
Scans of his kidney and lungs show that one of his tumours has shrunk and two others have not shown any further growth.
He is no longer taking the drugs and is being monitored every 12 weeks with scans.
Mr Waite said his daughters have teased him about being a guinea pig - and considered buying him some hay.
"I'm a very upbeat sort of bloke and I've been very lucky," he says.
"I feel very privileged to have had the opportunity to go on the trial."

As yet, nivolumab has only been approved for treating skin cancer and in June it became one of the fastest medicines ever approved for NHS use, in combination with ipilimumab, for the same cancer.
Nivolumab and ipilimumab both work by interrupting the chemical signals that cancers use to convince the immune system they are healthy tissue.
'Extend life'
Prof Kevin Harrington of the Institute of Cancer Research and consultant at the Royal Marsden Hospital in London, who led the head and neck cancer trial, said nivolumab could be a real "game changer" for patients with advanced head and neck cancer.
"This trial found that it can greatly extend life among a group of patients who have no existing treatment options, without worsening quality of life.
"Once it has relapsed or spread, head and neck cancer is extremely difficult to treat. So it's great news that these results indicate we now have a new treatment that can significantly extend life, and I'm keen to see it enter the clinic as soon as possible."
Prof Paul Workman, chief executive of The Institute of Cancer Research, said nivolumab was one of a new wave of immunotherapies that were beginning to have an impact across cancer treatment.
He added: "We hope regulators can work with the manufacturer to avoid delays in getting this drug to patients who have no effective treatment options left to them."
- Published1 June 2015
- Published26 September 2015
- Published2 July 2015
- Published1 June 2015