Sterilisation implant withdrawn from non-US sale
- Published

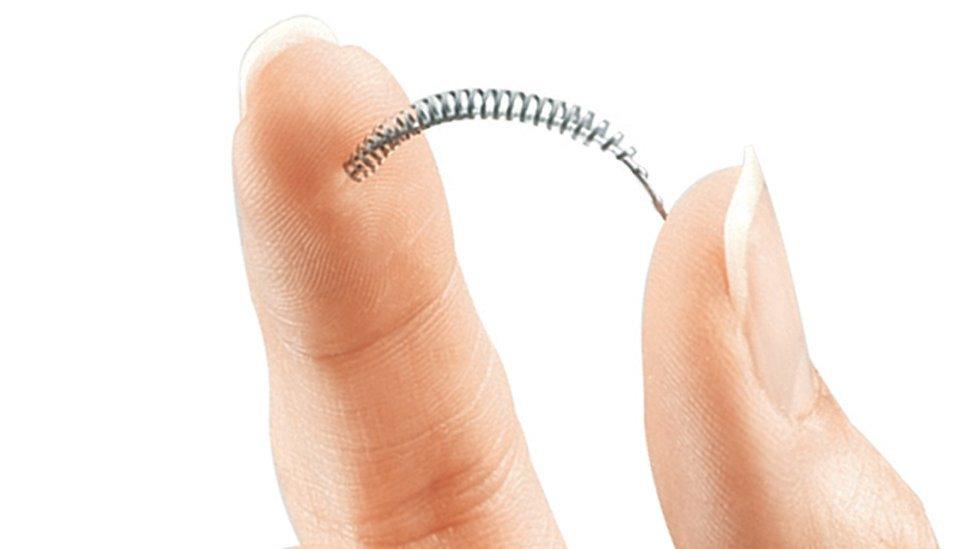
The coil is inserted into the fallopian tubes
Sales of a sterilisation device are being halted in all countries bar the US, weeks after the Victoria Derbyshire show reported it could cause problems.
The Essure implant, which is available on the NHS, has left some women in chronic pain, and some have even needed hysterectomies to remove it.
The pharmaceutical company Bayer said the decision to stop sales was being taken for commercial reasons.
The sale of the implants in the EU was temporarily suspended last month.
Bayer has asked hospitals in the UK not to use their existing stocks during this time.
It is a voluntary request and up to individual trusts to decide what to do.
'Felt like a burden'
The small coil implants, which are made of nickel and polyester (PET) fibres, are used as a sterilisation device to stop eggs reaching the womb.
They are inserted into the fallopian tubes where they are designed to trigger inflammation, causing scar tissue to build up and eventually block the tubes, known as a hysteroscopic sterilisation.
Victoria Dethier had a hysterectomy to remove the device
They can cause intense pain, and some women are thought to react badly to the nickel and plastic.
Because of the way the coils attach to the fallopian tubes, the only way to take them out is to remove a woman's fallopian tubes and often her uterus.
In other cases the device has been found to perforate a fallopian tube and fallen out, embedding itself elsewhere in the body.
Laura Linkson, who was fitted with the Essure device in 2013, told the BBC's Victoria Derbyshire programme the pain had left her suicidal.

Laura Linkson says she felt unable to look after her children because of the pain caused by the implant
"The device was sold to me as a simple and easy procedure. I was told that I'd be in and out of the doctor's office in 10 minutes and that there'd be no recovery time.
"I went from being a mum who was doing everything with her children, to a mum that was stuck in bed unable to move without pain, at some points being suicidal.
"I felt like I was a burden on everyone around me," she added.
Warning on packaging
The US is the biggest market for the product. More than 15,000 women have reported problems to the US Food and Drug Administration, which include "pain", "allergic reactions" and the coil moving to other parts of the body.
Last year, the FDA ordered Bayer to carry out long-term testing on Essure and put a warning on its packaging.
A spokeswoman for the FDA said it was aware Bayer was no longer marketing Essure outside the US and the company had confirmed it was committed to continuing the testing as ordered.
"The FDA has taken several steps to ensure the ongoing evaluation of Essure's safety and efficacy, as well as to educate healthcare professionals and women about the potential risks of using the device," she said.
A statement from Bayer said: "We would like to reassure all patients, especially those with Essure, as well as health professionals, that this decision has been taken for commercial reasons and is not linked to any problems with safety or with the quality of the product.
"According to our scientific assessment, the positive risk-benefit ratio of Essure remains unchanged.
"The safety and effectiveness of Essure is supported by over 10 years of scientific research and real-life clinical experience."
Watch the BBC's Victoria Derbyshire programme on weekdays between 09:00 and 11:00 on BBC Two and the BBC News channel.
- Published29 August 2017

- Published14 October 2015
- Published4 October 2015
