Hernia mesh complications 'affect more than 100,000'
- Published
David Ellis says complications following his hernia mesh operation nearly led him to take his own life
Up to 170,000 people who have had hernia mesh implants in England in the past six years could face complications, the BBC's Victoria Derbyshire programme has found.
In that time, there have been about 570,000 operations and some surgeons say the complication rate is 12-30%.
Some patients have been left unable to walk or work, others left suicidal.
The Medicines and Healthcare products Regulatory Agency backs the use of hernia mesh.
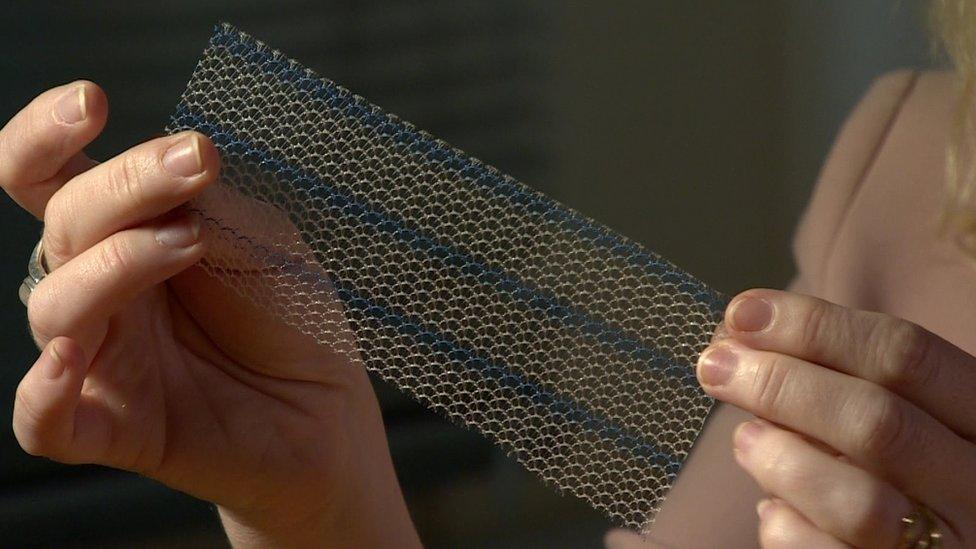
Mesh is used in more than 90,000 hernia operations in England each year
One in 10 people will develop a hernia.
The most common treatment involves a doctor pushing any bulging tissue back into the body and covering it with a piece of surgical mesh.
There have been between 90,000 and 100,000 hernia mesh operations in England each year since 2011-12.
And leading surgeons believe the complication rate is 12-30% - which means between 68,000 and 170,000 patients could have been adversely affected in the past six years.
Mesh has been used for hernia repairs since the 1990s, so the total number who have experienced complications since its introduction is thought to be much higher.
But NHS trusts in England have no consistent policy for guidelines on treatment or follow-up with patients.

Jen's story

Jen Coles had to use a cane to walk following her mesh implant
After Jen Coles, 34, had a mesh implant in January 2017, following her hernia, the operation left her with chronic pain.
"I was hunched over and walking as though I was elderly and I couldn't stand up straight and it hurt all the time," she said.
"I had a cane because I just felt really unsteady on my feet."
She has since had a repair operation to remove two large pieces of mesh.

Jen Coles was active, with lots of hobbies, before her mesh implant
Her family paid for the operation because they feared it would take years on the NHS.
Her overall mobility has improved, and the pain decreased, but she still struggles to do certain tasks.
"Sometimes, I just want to scream. It's maddening," she said.
"[Before the mesh implant] I was so active - running around commuting, kayaking, and now I can't pick up a sock from the floor."

Labour MP Owen Smith, who chairs the All Party Parliamentary Group on Surgical Mesh Implants, said he feared the UK could "potentially have another scandal on our hands".
He said the Medicines and Healthcare products Regulatory Agency (MHRA), whose job it is to ensure medical devices are safe, was not doing enough to listen to the experiences of patients affected.
"It reflects the flawed system we have in place," he said. "Neither the regulators or the manufacturers have to follow up on problems.
"Companies ultimately have to take some responsibility for this.
"It's not good for them to give this to the NHS and then they walk away with the NHS carrying any liability."
The MHRA told the BBC it had "not had any evidence which would lead us to alter our stance on surgical mesh for hernia repairs or other surgical procedures for which they are used".
"The decision to use mesh should be made between patient and clinician, recognising the benefits and risks," it added.
The Royal College of Surgeons said mesh implants were the "most effective way" to deal with a hernia.

Dr Ulrike Muschaweck has performed 3,000 mesh removals
Dr Ulrike Muschaweck, a leading hernia surgeon in the private sector, said she used a suture technique - instead of mesh - for most hernia operations but this method was dying out because young surgeons were rarely taught it.
She said she had performed 3,000 mesh removals because of chronic pain - after which only two of the patients had not gone on to become "pain-free".
Dr Suzy Elneil, a consultant urogynaecologist who was a leading voice in the successful campaign to halt the use of vaginal mesh on the NHS in England, estimated treating those who had experienced complications with hernia mesh could cost a minimum of £25,000 a patient.
This includes:
the removal of the mesh
a further operation to treat the hernia
follow-up care
It is similar to the predicted cost for those treated for vaginal mesh complications.
Watch the BBC's Victoria Derbyshire programme on weekdays between 09:00 and 11:00 BST on BBC Two and the BBC News channel in the UK and on iPlayer afterwards.
- Published26 June 2017

- Published26 June 2017

- Published27 November 2017