Cancer immunotherapy drug 'less toxic and prolongs life'
- Published

Derek, from Leicestershire, took part in a trial of pembrolizumab
An immunotherapy drug that could save some cancer patients from the ordeal of extreme chemotherapy may also help them live longer, researchers say.
In a trial, pembrolizumab kept head and neck cancers at bay for an average of two years - five times longer than under chemotherapy.
The patients also suffered far fewer side-effects.
Cases of head and neck cancers are rising in the UK and most are diagnosed late, when they are harder to treat.
'I can get on with my life'
Derek Kitcherside, 70, from Leicestershire, believes he wouldn't be here without pembrolizumab.
He was diagnosed with cancer of the voicebox in 2011. When he started coughing up blood three years later, after receiving standard treatment, he was told the cancer had spread to his lungs and was probably incurable.
Derek asked to go on a drug trial and travelled to London every three weeks for two years for treatment with pembrolizumab.
"My tumour was shrinking all the time and I felt a bit better every time I went," he said. "It made a huge difference to my life."
Regular scans show the disease is now stable and the tumour is still getting smaller.
"I'm very pleased I can get on with my life," Derek said.
"I don't think I'd be here without it."

Pembrolizumab is given via a drip
What is immunotherapy?
It is a treatment that does not kill cancer cells itself but instead stimulates the body's immune system to attack them.
Pembrolizumab is already being used to treat a wide range of advanced cancers, including melanoma - a type of skin cancer that spreads easily.
Experts believe the drug has the potential to treat many more.
When is it used?
Normally, immunotherapy is used after standard treatments like chemotherapy have failed but this trial, in 882 patients from 37 countries, suggests it should be used earlier - and for some people it should be the go-to option.
The drug is given to patients regularly through a drip when their cancer has returned or spread, and is considered incurable.
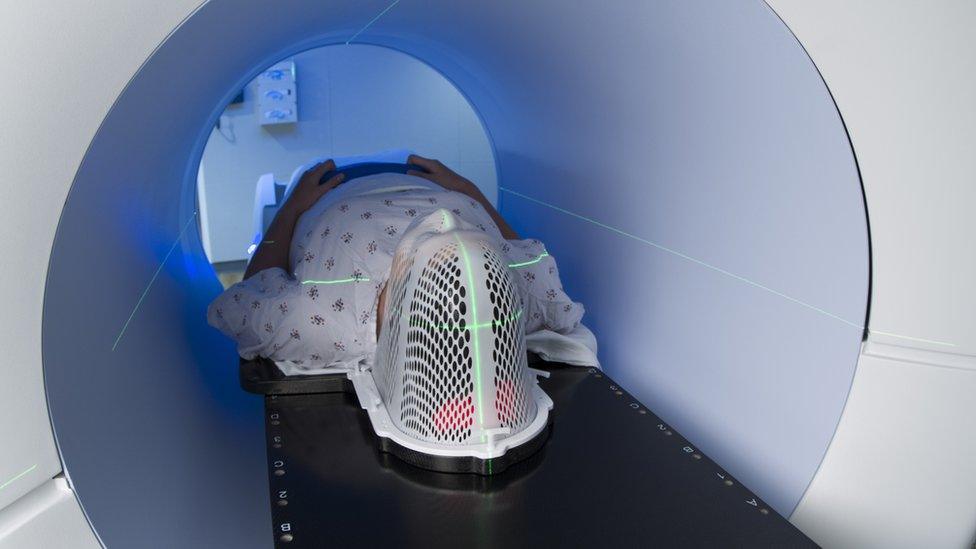
Patients with head and neck cancer must have regular scans to check progress of the disease
Why is it better than current treatment?
It is kinder, safer and can keep patients alive for longer, the study in The Lancet, external found.
But it may not work for everyone.
In people with advanced head and neck cancer who responded to the drug - one in four - their cancer shrank or stabilised for an average of 23 months.
By comparison, although more patients (36%) responded positively to standard chemotherapy treatment, the improvements lasted on average for only four and a half months.
Those with larger, more aggressive tumours were given the drug in combination with chemotherapy to help slow progress of the disease - which was kept in check for an average of seven months.
So who could benefit?
The trick is identifying people with tumours that will respond, says Prof Kevin Harrington, oncologist at The Royal Marsden NHS Founation Trust and professor of biological cancer therapies at the Institute of Cancer Research, who led the study,
A test for the presence of immune marker PD-L1 in the tumour means doctors can work this out.
Prof Harrington says approximately 85% of people with advanced or relapsed head and neck cancer would be eligible for pembrolizumab - around 1,300 patients a year.
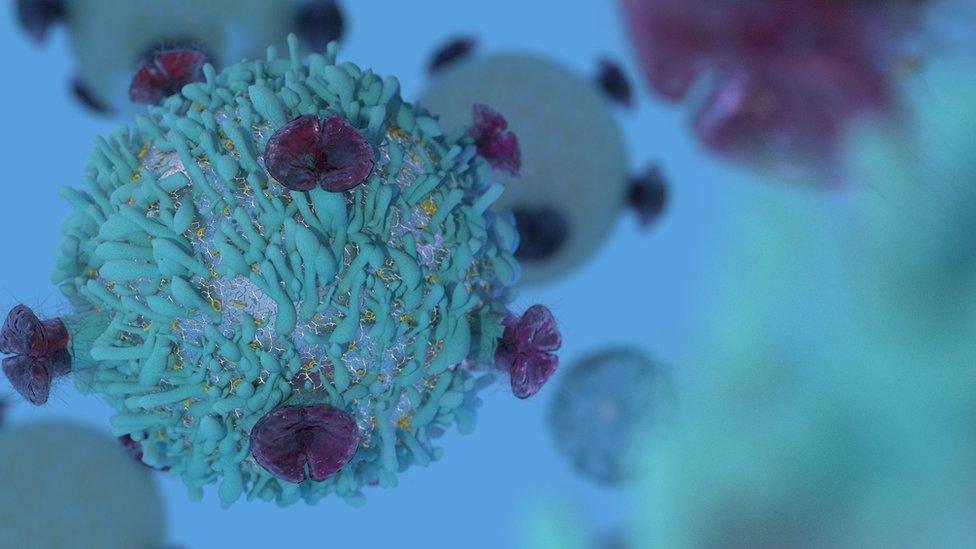
Immune cells can be targeted by immunotherapy treatment
Why is it kinder?
At the moment, the recommended treatment is a toxic combination of chemotherapy and antibody therapy, which often makes people feel very sick.
The extreme treatment can also cause damage to the kidneys, hands and feet.
Patients given the immunotherapy drug experienced far fewer side-effects.
"It's more sensible and less toxic - patients live longer and feel better," says Prof Harrington.
What does this mean for patients?
"This study is very exciting", says Prof Paul Workman, from the Institute of Cancer Research.
"Firstly because it shows that immunotherapy can have dramatic benefits for some patients with head and neck cancer when used as a first-line treatment, and secondly because the researchers have devised a test for picking out who is most likely to benefit."
Pembrolizumab is less toxic for patients than chemotherapy
He said all new drugs coming on to the market should be accompanied by a test to target their uses as precisely as possible.
In the US and the EU, pembrolizumab has been approved for use on its own and with chemotherapy for advanced head and neck cancer, but not yet in the UK.
The National Institute for Heath Care and Excellence is currently appraising pembrolizumab and it could be approved for use on the NHS by next summer.
Facts on head and neck cancer
Around 12,000 people in the UK are diagnosed with head and neck cancer each year
Half are diagnosed at stage III or IV when it is hard to treat
Most cases are linked to smoking and alcohol but the recent rise in cases seems to be linked to HPV (human papillomavirus)
This virus infects the skin and cells lining the inside of the body and can be spread through close skin-to-skin contact
Boys and girls are now being vaccinated against HPV at school, but it will be several decades before HPV stops being a risk factor
- Published9 October 2016

- Published19 April 2016
- Published28 September 2019
- Published2 June 2018
