Coronavirus: Potential drug treatment starts UK trials
- Published
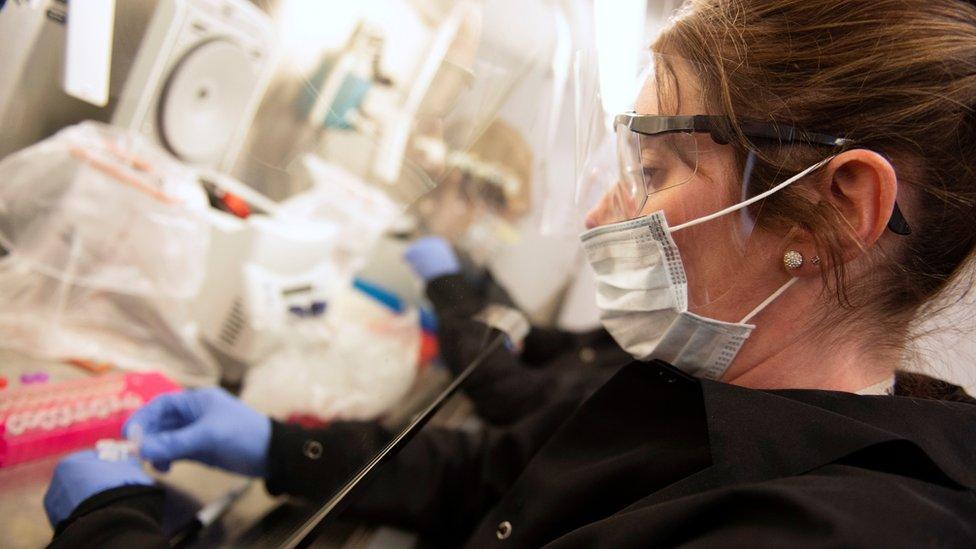
Trials have begun globally of a handful of potential treatments
A drug that could help treat coronavirus is to be trialled on a small number of patients in England and Scotland.
The studies, which have been fast-tracked by the government, will initially involve 15 NHS centres.
In the absence of a known treatment for the virus, a handful of experimental drugs are being tested globally.
The drug, known as remdesivir, is manufactured by the pharmaceutical company Gilead.
Two studies are to be carried out in the UK - one on patients with moderate symptoms, and one on those who are in a serious condition.
Trials are already underway in China and the US, with the first results expected in the coming weeks.
The UK trials will be based in England and Scotland and overseen by Dr Andrew Ustianowski, a consultant in infectious diseases.

A SIMPLE GUIDE: How do I protect myself?
AVOIDING CONTACT: The rules on self-isolation and exercise
LOOK-UP TOOL: Check cases in your area
MAPS AND CHARTS: Visual guide to the outbreak
VIDEO: The 20-second hand wash

He's spent the past couple of weeks working full time helping treat COVID-19 patients and has seen first-hand how sick patients can become.
"What we really need, and what we really want, is a specific treatment against Coronavirus that delays the infection, treats the infection, and hopefully makes people better.
"I think this drug is promising in the laboratory, and we're hopeful it will be as promising in humans.
"In my heart I'm hopeful, but we do need studies such as this to work out how well it works and how best to use it."
Anti-viral drugs
Gilead specialises in producing anti-viral medications.
Hilary Hutton-Squire, the company's General Manager in the UK and Ireland, says the work behind this drug stretches back over ten years.
"For about a decade we've been looking at what we call emerging viruses, looking at viruses that aren't a problem yet but could be in the future.
"Coronaviruses are an important category of virus because when we've seen them jump from animals to humans previously they've caused a lot of problems as with SARS and MERS,.
"So remdesivir was a product we had looked at against SARS and MERS and seen that it had some activity, and that's why we thought it was really important to see if it has a role to play in treating patients with COVID-19 as quickly as we can."
The UK regulator, the Medicines and Healthcare Products Regulatory Agency (MHRA), said it was ready to prioritise and provide any assistance in response to Covid-19, in line with government priorities.
"We have procedures for rapid scientific advice, reviews and approvals and are ready to support manufacturers, researchers and other regulators," said Dr Siu Ping Lam.
Remdesivir has been considered as a potential treatment for Ebola.
The drug is designed to interfere with the way a virus reproduces, thereby stopping it from multiplying inside the body.
With no approved therapies for coronavirus infection, hopes rest on speeding up the approval process for drugs that show promise in fighting the disease.
US researchers have begun a trial trial to see if the malaria drug, chloroquine, will help treat coronavirus.
- Published31 March 2020

- Published27 July 2020